Blue-Light Receptors for Optogenetics
- PMID: 29984995
- PMCID: PMC6500593
- DOI: 10.1021/acs.chemrev.8b00163
Blue-Light Receptors for Optogenetics
Abstract
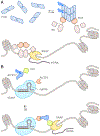
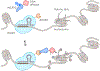
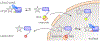
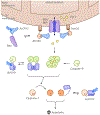
Sensory photoreceptors underpin light-dependent adaptations of organismal physiology, development, and behavior in nature. Adapted for optogenetics, sensory photoreceptors become genetically encoded actuators and reporters to enable the noninvasive, spatiotemporally accurate and reversible control by light of cellular processes. Rooted in a mechanistic understanding of natural photoreceptors, artificial photoreceptors with customized light-gated function have been engineered that greatly expand the scope of optogenetics beyond the original application of light-controlled ion flow. As we survey presently, UV/blue-light-sensitive photoreceptors have particularly allowed optogenetics to transcend its initial neuroscience applications by unlocking numerous additional cellular processes and parameters for optogenetic intervention, including gene expression, DNA recombination, subcellular localization, cytoskeleton dynamics, intracellular protein stability, signal transduction cascades, apoptosis, and enzyme activity. The engineering of novel photoreceptors benefits from powerful and reusable design strategies, most importantly light-dependent protein association and (un)folding reactions. Additionally, modified versions of these same sensory photoreceptors serve as fluorescent proteins and generators of singlet oxygen, thereby further enriching the optogenetic toolkit. The available and upcoming UV/blue-light-sensitive actuators and reporters enable the detailed and quantitative interrogation of cellular signal networks and processes in increasingly more precise and illuminating manners.
Figures





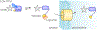



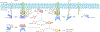

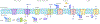
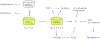
References
-
- Möglich A; Yang X; Ayers RA; Moffat K Structure and Function of Plant Photoreceptors. Annu. Rev. Plant Biol 2010, 61, 21–47. - PubMed
-
- Hegemann P Algal Sensory Photoreceptors. Annu. Rev. Plant Biol. 2008, 59, 167–189. - PubMed
-
- Sorigué D; Légeret B; Cuiné S; Blangy S; Moulin S; Billon E; Richaud P; Brugière S; Couté Y; Nurizzo D; et al. An Algal Photoenzyme Converts Fatty Acids to Hydrocarbons. Science 2017, 357, 903–907. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

