Bowman's capsule provides a protective niche for podocytes from cytotoxic CD8+ T cells
- PMID: 29985168
- PMCID: PMC6063505
- DOI: 10.1172/JCI97879
Bowman's capsule provides a protective niche for podocytes from cytotoxic CD8+ T cells
Abstract
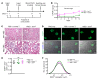
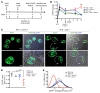
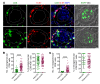
T cells play a key role in immune-mediated glomerulonephritis, but how cytotoxic T cells interact with podocytes remains unclear. To address this, we injected EGFP-specific CD8+ T cells from just EGFP death inducing (Jedi) mice into transgenic mice with podocyte-specific expression of EGFP. In healthy mice, Jedi T cells could not access EGFP+ podocytes. Conversely, when we induced nephrotoxic serum nephritis (NTSN) and injected Jedi T cells, EGFP+ podocyte transgenic mice showed enhanced proteinuria and higher blood urea levels. Morphometric analysis showed greater loss of EGFP+ podocytes, which was associated with severe crescentic and necrotizing glomerulonephritis. Notably, only glomeruli with disrupted Bowman's capsule displayed massive CD8+ T cell infiltrates that were in direct contact with EGFP+ podocytes, causing their apoptosis. Thus, under control conditions with intact Bowman's capsule, podocytes are not accessible to CD8+ T cells. However, breaches in Bowman's capsule, as also noted in human crescentic glomerulonephritis, allow access of CD8+ T cells to the glomerular tuft and podocytes, resulting in their destruction. Through these mechanisms, a potentially reversible glomerulonephritis undergoes an augmentation process to a rapidly progressive glomerulonephritis, leading to end-stage kidney disease. Translating these mechanistic insights to human crescentic nephritis should direct future therapeutic interventions at blocking CD8+ T cells, especially in progressive stages of rapidly progressive glomerulonephritis.
Keywords: Chronic kidney disease; Immunology; Nephrology; T cells.
Conflict of interest statement
Figures




Comment in
-
CD8+ cells and glomerular crescent formation: outside-in as well as inside-out.J Clin Invest. 2018 Aug 1;128(8):3231-3233. doi: 10.1172/JCI122045. Epub 2018 Jul 9. J Clin Invest. 2018. PMID: 29985169 Free PMC article.
-
The Bowman's shield: a tribute to translational science and Detlef Schlöndorff.Kidney Int. 2018 Sep;94(3):448-450. doi: 10.1016/j.kint.2018.07.001. Epub 2018 Jul 10. Kidney Int. 2018. PMID: 30006006 No abstract available.
-
Breaches in the Bowman's capsule and CD8+ T cell infiltration in crescentic GN.Nat Rev Nephrol. 2018 Oct;14(10):597. doi: 10.1038/s41581-018-0046-y. Nat Rev Nephrol. 2018. PMID: 30042436 No abstract available.
References
-
- Jennette JC, Thomas DB. Crescentic glomerulonephritis. Nephrol Dial Transplant. 2001;16(Suppl 6):80–82. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

