Clarin-1 gene transfer rescues auditory synaptopathy in model of Usher syndrome
- PMID: 29985171
- PMCID: PMC6063508
- DOI: 10.1172/JCI94351
Clarin-1 gene transfer rescues auditory synaptopathy in model of Usher syndrome
Abstract
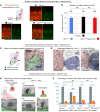
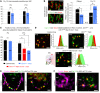
Clarin-1, a tetraspan-like membrane protein defective in Usher syndrome type IIIA (USH3A), is essential for hair bundle morphogenesis in auditory hair cells. We report a new synaptic role for clarin-1 in mouse auditory hair cells elucidated by characterization of Clrn1 total (Clrn1ex4-/-) and postnatal hair cell-specific conditional (Clrn1ex4fl/fl Myo15-Cre+/-) knockout mice. Clrn1ex4-/- mice were profoundly deaf, whereas Clrn1ex4fl/fl Myo15-Cre+/- mice displayed progressive increases in hearing thresholds, with, initially, normal otoacoustic emissions and hair bundle morphology. Inner hair cell (IHC) patch-clamp recordings for the 2 mutant mice revealed defective exocytosis and a disorganization of synaptic F-actin and CaV1.3 Ca2+ channels, indicative of a synaptopathy. Postsynaptic defects were also observed, with an abnormally broad distribution of AMPA receptors associated with a loss of afferent dendrites and defective electrically evoked auditory brainstem responses. Protein-protein interaction assays revealed interactions between clarin-1 and the synaptic CaV1.3 Ca2+ channel complex via the Cavβ2 auxiliary subunit and the PDZ domain-containing protein harmonin (defective in Usher syndrome type IC). Cochlear gene therapy in vivo, through adeno-associated virus-mediated Clrn1 transfer into hair cells, prevented the synaptic defects and durably improved hearing in Clrn1ex4fl/fl Myo15-Cre+/- mice. Our results identify clarin-1 as a key organizer of IHC ribbon synapses, and suggest new treatment possibilities for USH3A patients.
Keywords: Calcium channels; Gene therapy; Neuroscience; Synapses.
Conflict of interest statement
Figures

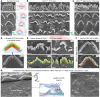
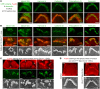
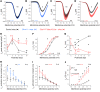
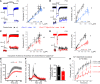






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

