Multidimensional communication in the microenvirons of glioblastoma
- PMID: 29985475
- PMCID: PMC6425928
- DOI: 10.1038/s41582-018-0025-8
Multidimensional communication in the microenvirons of glioblastoma
Abstract
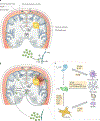
Glioblastomas are heterogeneous and invariably lethal tumours. They are characterized by genetic and epigenetic variations among tumour cells, which makes the development of therapies that eradicate all tumour cells challenging and currently impossible. An important component of glioblastoma growth is communication with and manipulation of other cells in the brain environs, which supports tumour progression and resistance to therapy. Glioblastoma cells recruit innate immune cells and change their phenotype to support tumour growth. Tumour cells also suppress adaptive immune responses, and our increasing understanding of how T cells access the brain and how the tumour thwarts the immune response offers new strategies for mobilizing an antitumour response. Tumours also subvert normal brain cells - including endothelial cells, neurons and astrocytes - to create a microenviron that favours tumour success. Overall, after glioblastoma-induced phenotypic modifications, normal cells cooperate with tumour cells to promote tumour proliferation, invasion of the brain, immune suppression and angiogenesis. This glioblastoma takeover of the brain involves multiple modes of communication, including soluble factors such as chemokines and cytokines, direct cell-cell contact, extracellular vesicles (including exosomes and microvesicles) and connecting nanotubes and microtubes. Understanding these multidimensional communications between the tumour and the cells in its environs could open new avenues for therapy.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures



References
-
- Stupp R et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10, 459–466 (2009). - PubMed
-
- Jhaveri N, Chen TC & Hofman FM Tumor vasculature and glioma stem cells: contributions to glioma progression. Cancer Lett 380, 545–551 (2016). - PubMed
-
- See AP, Parker JJ & Waziri A The role of regulatory T cells and microglia in glioblastoma-associated immunosuppression. J. Neurooncol 23, 405–412 (2015). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

