Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery
- PMID: 29985479
- PMCID: PMC6078803
- DOI: 10.1038/nbt.4181
Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery
Abstract
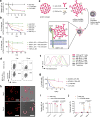
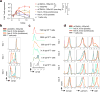
Adoptive cell therapy (ACT) with antigen-specific T cells has shown remarkable clinical success; however, approaches to safely and effectively augment T cell function, especially in solid tumors, remain of great interest. Here we describe a strategy to 'backpack' large quantities of supporting protein drugs on T cells by using protein nanogels (NGs) that selectively release these cargos in response to T cell receptor activation. We designed cell surface-conjugated NGs that responded to an increase in T cell surface reduction potential after antigen recognition and limited drug release to sites of antigen encounter, such as the tumor microenvironment. By using NGs that carried an interleukin-15 super-agonist complex, we demonstrated that, relative to systemic administration of free cytokines, NG delivery selectively expanded T cells 16-fold in tumors and allowed at least eightfold higher doses of cytokine to be administered without toxicity. The improved therapeutic window enabled substantially increased tumor clearance by mouse T cell and human chimeric antigen receptor (CAR)-T cell therapy in vivo.
Conflict of interest statement
D.J.I., L.T., and Y.Z. are inventors on licensed patents related to the technology described in this manuscript. D.J.I. is a co-founder of Torque Therapeutics, which licensed patents related to this technology.
Figures




References
-
- Gill S, June CH. Going viral: chimeric antigen receptor T cell therapy for hematological malignancies. Immunol Rev. 2015;263:68–89. - PubMed
-
- Corrigan-Curay J, Kiem HP, Baltimore D, O'Reilly M, Brentjens RJ, Cooper L, Forman S, Gottschalk S, Greenberg P, Junghans R, Heslop H, Jensen M, Mackall C, June C, Press O, Powell D, Ribas A, Rosenberg S, Sadelain M, Till B, Patterson AP, Jambou RC, Rosenthal E, Gargiulo L, Montgomery M, Kohn DB. T cell immunotherapy: looking forward. Mol Ther. 2014;22:1564–1574. - PMC - PubMed
-
- Johnson LA, Scholler J, Ohkuri T, Kosaka A, Patel PR, McGettigan SE, Nace AK, Dentchev T, Thekkat P, Loew A, Boesteanu AC, Cogdill AP, Chen T, Fraietta JA, Kloss CC, Posey AD, Engels B, Singh R, Ezell T, Idamakanti N, Ramones MH, Li N, Zhou L, Plesa G, Seykora JT, Okada H, June CH, Brogdon JL, Maus MV. Rational development and characterization of humanized anti–EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med. 2015;7:275ra222. - PMC - PubMed
-
- Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, Tagaya Y, Rosenberg SA, Waldmann TA, Restifo NP. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T Cells. Proc Natl Acad Sci. 2004;101:1969–1974. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

