High Viral Diversity and Mixed Infections in Cerebral Spinal Fluid From Cases of Varicella Zoster Virus Encephalitis
- PMID: 29986093
- PMCID: PMC6173578
- DOI: 10.1093/infdis/jiy358
High Viral Diversity and Mixed Infections in Cerebral Spinal Fluid From Cases of Varicella Zoster Virus Encephalitis
Abstract
Background: Varicella zoster virus (VZV) may cause encephalitis, both with and without rash. Here we investigate whether viruses recovered from the central nervous system (CNS; encephalitis or meningitis) differ genetically from those recovered from non-CNS samples.
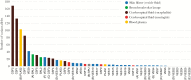
Methods: Enrichment-based deep sequencing of 45 VZV genomes from cerebral spinal fluid (CSF), plasma, bronchoalveolar lavage (BAL), and vesicles was carried out with samples collected from 34 patients with and without VZV infection of the CNS.
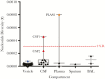
Results: Viral sequences from multiple sites in the same patient were identical at the consensus level. Virus from vesicle fluid and CSF in cases of meningitis showed low-level diversity. By contrast, plasma, BAL, and encephalitis had higher numbers of variant alleles. Two CSF-encephalitis samples had high genetic diversity, with variant frequency patterns typical of mixed infections with different clades.
Conclusions: Low viral genetic diversity in vesicle fluid is compatible with previous observations that VZV skin lesions arise from single or low numbers of virions. A similar result was observed in VZV from cases of VZV meningitis, a generally self-limiting infection. CSF from cases of encephalitis had higher diversity with evidence for mixed clade infections in 2 cases. We hypothesize that reactivation from multiple neurons may contribute to the pathogenesis of VZV encephalitis.
Figures



References
-
- Arvin AM, Moffat JF, Redman R. Varicella-zoster virus: aspects of pathogenesis and host response to natural infection and varicella vaccine. Adv Virus Res 1996; 46:263–309. - PubMed
-
- Burke BL, Steele RW, Beard OW, Wood JS, Cain TD, Marmer DJ. Immune responses to varicella-zoster in the aged. Arch Intern Med 1982; 142:291–3. - PubMed
-
- Persson A, Bergström T, Lindh M, Namvar L, Studahl M. Varicella-zoster virus CNS disease–viral load, clinical manifestations and sequels. J Clin Virol 2009; 46:249–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- IS-HPU-1112-10117/DH_/Department of Health/United Kingdom
- RP-PG-0108-10048/DH_/Department of Health/United Kingdom
- G0700814/MRC_/Medical Research Council/United Kingdom
- 12/205/28/DH_/Department of Health/United Kingdom
- BB/P007562/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

