Detection of the Mitochondrial Membrane Potential by the Cationic Dye JC-1 in L1210 Cells with Massive Overexpression of the Plasma Membrane ABCB1 Drug Transporter
- PMID: 29986516
- PMCID: PMC6073605
- DOI: 10.3390/ijms19071985
Detection of the Mitochondrial Membrane Potential by the Cationic Dye JC-1 in L1210 Cells with Massive Overexpression of the Plasma Membrane ABCB1 Drug Transporter
Abstract
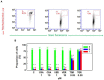
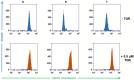
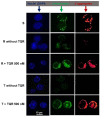
JC-1, a cationic fluorescent dye when added to living cells, is known to be localized exclusively in mitochondria, particularly in good physiological conditions characterized by sufficient mitochondrial membrane potential (ΔΨ). The accumulation of JC-1 in these organelles leads to the formation J-aggregates (with a specific red fluorescence emission maximum at 590 nm), which is in addition to the typical green fluorescence of J-monomers (emission maximum of ∼529 nm). The lack of mitochondrial ΔΨ leads to the depression of JC-1 mitochondrial accumulation and a decrease in J-aggregate formation. Therefore, the ratio between the red and green fluorescence of cells loaded with JC-1 is often used for the detection of the mitochondrial membrane potential. However, JC-1 represents a suitable substrate of the multidrug transporter P-glycoprotein (P-gp). Therefore, the depression of the JC-1 content in intracellular space and particularly in the mitochondria to a level that is inefficient for J-aggregate formation could be expected in P-gp-positive cells. In the current paper, we proved this behavior on parental P-gp-negative L1210 (S) cells and their P-gp-positive variants obtained by either selection with vincristine (R) or transfection with the human gene encoding P-gp (T). P-glycoprotein inhibitors cyclosporine A and verapamil fail to restore JC-1 loading of the R and T cells to an extent similar to that observed in S cells. In contrast, the noncompetitive high affinity P-gp inhibitor tariquidar fully restored JC-1 accumulation and the presence of the typical red fluorescence of J-aggregates. In the presence of tariquidar, measurement of the JC-1 fluorescence revealed similar levels of mitochondrial membrane potential in P-gp-negative (S) and P-gp-positive cells (R and T).
Keywords: JC-1; P-glycoprotein; mitochondrial membrane potential; multidrug resistance; tariquidar.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ortiz-Lazareno P.C., Bravo-Cuellar A., Lerma-Diaz J.M., Jave-Suarez L.F., Aguilar-Lemarroy A., Dominguez-Rodriguez J.R., Gonzalez-Ramella O., de Celis R., Gomez-Lomeli P., Hernandez-Flores G. Sensitization of u937 leukemia cells to doxorubicin by the mg132 proteasome inhibitor induces an increase in apoptosis by suppressing NF-κB and mitochondrial membrane potential loss. Cancer Cell Int. 2014;14:13. doi: 10.1186/1475-2867-14-13. - DOI - PMC - PubMed
-
- Salvioli S., Ardizzoni A., Franceschi C., Cossarizza A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: Implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997;411:77–82. doi: 10.1016/S0014-5793(97)00669-8. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

