Bioavailability Enhancement of Poorly Water-Soluble Drugs via Nanocomposites: Formulation⁻Processing Aspects and Challenges
- PMID: 29986543
- PMCID: PMC6160929
- DOI: 10.3390/pharmaceutics10030086
Bioavailability Enhancement of Poorly Water-Soluble Drugs via Nanocomposites: Formulation⁻Processing Aspects and Challenges
Abstract
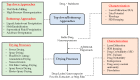
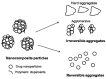
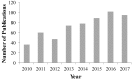
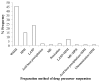
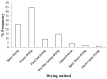
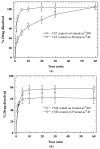
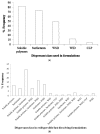
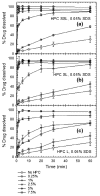
Drug nanoparticles embedded in a dispersant matrix as a secondary phase, i.e., drug-laden nanocomposites, offer a versatile delivery platform for enhancing the dissolution rate and bioavailability of poorly water-soluble drugs. Drug nanoparticles are prepared by top-down, bottom-up, or combinative approaches in the form of nanosuspensions, which are subsequently dried to prepare drug-laden nanocomposites. In this comprehensive review paper, the term “nanocomposites” is used in a broad context to cover drug nanoparticle-laden intermediate products in the form of powders, cakes, and extrudates, which can be incorporated into final oral solid dosages via standard pharmaceutical unit operations, as well as drug nanoparticle-laden strip films. The objective of this paper is to review studies from 2012⁻2017 in the field of drug-laden nanocomposites. After a brief overview of the various approaches used for preparing drug nanoparticles, the review covers drying processes and dispersant formulations used for the production of drug-laden nanocomposites, as well as various characterization methods including quiescent and agitated redispersion tests. Traditional dispersants such as soluble polymers, surfactants, other water-soluble dispersants, and water-insoluble dispersants, as well as novel dispersants such as wet-milled superdisintegrants, are covered. They exhibit various functionalities such as drug nanoparticle stabilization, mitigation of aggregation, formation of nanocomposite matrix⁻film, wettability enhancement, and matrix erosion/disintegration. Major challenges such as nanoparticle aggregation and poor redispersibility that cause inferior dissolution performance of the drug-laden nanocomposites are highlighted. Literature data are analyzed in terms of usage frequency of various drying processes and dispersant classes. We provide some engineering considerations in comparing drying processes, which could account for some of the diverging trends in academia vs. industrial practice. Overall, this review provides rationale and guidance for drying process selection and robust nanocomposite formulation development, with insights into the roles of various classes of dispersants.
Keywords: BCS Class II drugs; aggregates; dissolution enhancement; drug nanosuspensions; formulation; nanocomposites; redispersion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

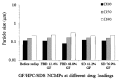
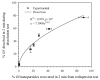
References
-
- Lipinski C. Poor aqueous solubility—An industry wide problem in drug discovery. Am. Pharm. Rev. 2002;5:82–85.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

