The BabySeq project: implementing genomic sequencing in newborns
- PMID: 29986673
- PMCID: PMC6038274
- DOI: 10.1186/s12887-018-1200-1
The BabySeq project: implementing genomic sequencing in newborns
Abstract
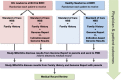
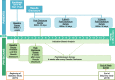
Background: The greatest opportunity for lifelong impact of genomic sequencing is during the newborn period. The "BabySeq Project" is a randomized trial that explores the medical, behavioral, and economic impacts of integrating genomic sequencing into the care of healthy and sick newborns.
Methods: Families of newborns are enrolled from Boston Children's Hospital and Brigham and Women's Hospital nurseries, and half are randomized to receive genomic sequencing and a report that includes monogenic disease variants, recessive carrier variants for childhood onset or actionable disorders, and pharmacogenomic variants. All families participate in a disclosure session, which includes the return of results for those in the sequencing arm. Outcomes are collected through review of medical records and surveys of parents and health care providers and include the rationale for choice of genes and variants to report; what genomic data adds to the medical management of sick and healthy babies; and the medical, behavioral, and economic impacts of integrating genomic sequencing into the care of healthy and sick newborns.
Discussion: The BabySeq Project will provide empirical data about the risks, benefits and costs of newborn genomic sequencing and will inform policy decisions related to universal genomic screening of newborns.
Trial registration: The study is registered in ClinicalTrials.gov Identifier: NCT02422511 . Registration date: 10 April 2015.
Keywords: Ethical, legal, social implications; Methods; Newborn screening; Newborn sequencing; Randomized trial; Whole exome sequencing.
Conflict of interest statement
Ethics approval and consent to participate
The BCH, BWH, and BCM IRBs have approved this study. All parents provided consent for their child and themselves to participate in the study. All health care providers consented to the study by completing the survey, which constitutes consent.
Consent for publication
Not applicable.
Competing interests
Authors have declared competing interest as follows:
HLR is employed by Partners Healthcare and Broad Institute that offer fee-based clinical sequencing. TWY is a founder of and consultant for Claritas Genomics, a diagnostic company for children with complex genetic disorders. RCG receives compensation for speaking or consultation from AIA, GenePeeks, Helix, Illumina, Ohana, Prudential, and Veritas, and is co-founder and advisor to Genome Medical, Inc. IAH, PBA, OCB, KDC, SF, LAF, CAG, JBK, RCL, HLL, ALM, RBP, PJP, SP, TSS, SEW, and AHB declare no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Green RC, Rehm HL, Kohane IS: Clinical genome sequencing. In: Genomic and Personalized Medicine.Volume 1, 2nd edn Edited by Ginsburg GS, Willard HF. San Diego: Elsevier Inc.; 2013: 102–122.
-
- Medical genetics laboratories: Whole genome laboratory (WGL) [http://www.bcm.edu/geneticlabs/index.cfm?PMID=21319].
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous