CO-dependent hydrogen production by the facultative anaerobe Parageobacillus thermoglucosidasius
- PMID: 29986719
- PMCID: PMC6036681
- DOI: 10.1186/s12934-018-0954-3
CO-dependent hydrogen production by the facultative anaerobe Parageobacillus thermoglucosidasius
Abstract
Background: The overreliance on dwindling fossil fuel reserves and the negative climatic effects of using such fuels are driving the development of new clean energy sources. One such alternative source is hydrogen (H2), which can be generated from renewable sources. Parageobacillus thermoglucosidasius is a facultative anaerobic thermophilic bacterium which is frequently isolated from high temperature environments including hot springs and compost.
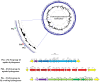
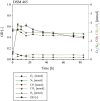
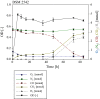
Results: Comparative genomics performed in the present study showed that P. thermoglucosidasius encodes two evolutionary distinct H2-uptake [Ni-Fe]-hydrogenases and one H2-evolving hydrogenases. In addition, genes encoding an anaerobic CO dehydrogenase (CODH) are co-localized with genes encoding a putative H2-evolving hydrogenase. The co-localized of CODH and uptake hydrogenase form an enzyme complex that might potentially be involved in catalyzing the water-gas shift reaction (CO + H2O → CO2 + H2) in P. thermoglucosidasius. Cultivation of P. thermoglucosidasius DSM 2542T with an initial gas atmosphere of 50% CO and 50% air showed it to be capable of growth at elevated CO concentrations (50%). Furthermore, GC analyses showed that it was capable of producing hydrogen at an equimolar conversion with a final yield of 1.08 H2/CO.
Conclusions: This study highlights the potential of the facultative anaerobic P. thermoglucosidasius DSM 2542T for developing new strategies for the biohydrogen production.
Keywords: Biohydrogen production; Carbon monoxide dehydrogenase; Hydrogenase; Parageobacillus thermoglucosidasius; Water-gas shift reaction.
Figures





References
-
- International Energy Agency. World Energy Outlook 2017. 2017. https://www.iea.org/weo2017. Accessed 26 Mar 2018.
-
- Nikolaidis P, Poullikkas A. A comparative overview of hydrogen production processes. Renew Sustain Energy Rev. 2017;67:597–611. doi: 10.1016/j.rser.2016.09.044. - DOI
-
- Hosseini S, Wahid M. Hydrogen production from renewable and sustainable energy resources: promising green energy carrier for clean development. Renew Sustain Energy Rev. 2016;57:850–866. doi: 10.1016/j.rser.2015.12.112. - DOI
-
- Claassen PAM, van Lier JB, Lopez Contreras AM, van Niel EWJ, Sijtsma L, Stams AJM, et al. Utilisation of biomass for the supply of energy carriers. Appl Microbiol Biotechnol. 1999;52:741–755. doi: 10.1007/s002530051586. - DOI
-
- Kothari R, Buddhi D, Sawhney RL. Comparison of environmental and economic aspects of various hydrogen production methods. Renew Sustain Energy Rev. 2008;12:553–563. doi: 10.1016/j.rser.2006.07.012. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

