Intra-operative spectroscopic assessment of surgical margins during breast conserving surgery
- PMID: 29986750
- PMCID: PMC6038277
- DOI: 10.1186/s13058-018-1002-2
Intra-operative spectroscopic assessment of surgical margins during breast conserving surgery
Abstract
Background: In over 20% of breast conserving operations, postoperative pathological assessment of the excised tissue reveals positive margins, requiring additional surgery. Current techniques for intra-operative assessment of tumor margins are insufficient in accuracy or resolution to reliably detect small tumors. There is a distinct need for a fast technique to accurately identify tumors smaller than 1 mm2 in large tissue surfaces within 30 min.
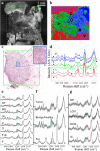
Methods: Multi-modal spectral histopathology (MSH), a multimodal imaging technique combining tissue auto-fluorescence and Raman spectroscopy was used to detect microscopic residual tumor at the surface of the excised breast tissue. New algorithms were developed to optimally utilize auto-fluorescence images to guide Raman measurements and achieve the required detection accuracy over large tissue surfaces (up to 4 × 6.5 cm2). Algorithms were trained on 91 breast tissue samples from 65 patients.
Results: Independent tests on 121 samples from 107 patients - including 51 fresh, whole excision specimens - detected breast carcinoma on the tissue surface with 95% sensitivity and 82% specificity. One surface of each uncut excision specimen was measured in 12-24 min. The combination of high spatial-resolution auto-fluorescence with specific diagnosis by Raman spectroscopy allows reliable detection even for invasive carcinoma or ductal carcinoma in situ smaller than 1 mm2.
Conclusions: This study provides evidence that this multimodal approach could provide an objective tool for intra-operative assessment of breast conserving surgery margins, reducing the risk for unnecessary second operations.
Keywords: Auto-fluorescence; Breast cancer; Intra-operative margin evaluation; Raman spectroscopy.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval was granted through the Nottingham Health Science Biobank (NHSB, REC reference 15/NW/0685) and informed consent was obtained from all patients.
Competing interests
The authors have no competing interests to declare. IN has filed a patent application related to MSH.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures






Comment in
-
Can novel technologies improve breast conserving surgery?Breast Cancer Res. 2018 Aug 3;20(1):85. doi: 10.1186/s13058-018-1016-9. Breast Cancer Res. 2018. PMID: 30075805 Free PMC article. No abstract available.
References
-
- Moran MS, Schmitt SJ, Giuliano AE, Harris JR, Khan SA, Horton J, et al. Society of Surgical Oncology – American Society for Radiation Oncology consensus guideline on margins for breast conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Ann Surg Oncol. 2014;21:704–716. doi: 10.1245/s10434-014-3481-4. - DOI - PubMed
-
- Morrow M, Van Zee KJ, Solin LJ, Houssami N, Chavez-MacGregor M, Harris JR, et al. Society of Surgical Oncology – American Society for Radiation Oncology – American Society of Clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. J Clin Oncol. 2016;34(33):4040–4046. doi: 10.1200/JCO.2016.68.3573. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

