Epithelial Sodium Channel in Aldosterone-Induced Endothelium Stiffness and Aortic Dysfunction
- PMID: 29987101
- PMCID: PMC6202124
- DOI: 10.1161/HYPERTENSIONAHA.118.11339
Epithelial Sodium Channel in Aldosterone-Induced Endothelium Stiffness and Aortic Dysfunction
Abstract
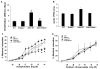
Enhanced activation of the endothelial mineralocorticoid receptor contributes to the development of arterial stiffness, which is an independent predictor of cardiovascular disease. Previously, we showed that enhanced endothelium mineralocorticoid receptor signaling in female mice prompts expression and translocation of the α-subunit of the epithelial sodium channel to the endothelial cell (EC) surface (EnNaC) inducing vascular fibrosis and stiffness. Further, amiloride, an epithelial sodium channel antagonist, inhibits vascular fibrosis, remodeling, and stiffness induced by feeding a Western diet high in saturated fat and refined carbohydrates. However, how this occurs remains unknown. Thereby, we hypothesized that endothelial cell-specific EnNaC activation is necessary for aldosterone-mediated endothelium stiffness. To address this notion, EnNaC α-subunit knockout (EnNaC-/-) and wild-type littermate female mice were administrated aldosterone (250 µg/kg per day) via osmotic minipumps for 3 weeks beginning at 25 to 28 weeks of age. In isolated mouse endothelial cells, inward sodium currents were significantly reduced in amiloride controls, as well as in EnNaC-/-. Likewise, aldosterone-induced endothelium stiffness was increased and endothelium-dependent relaxation less in EnNaC-/- versus wild-type. Further, EnNaC-/- mice exhibited attenuated responses to aldosterone infusion, including aortic endoplasmic reticulum stress, endothelium nitric oxide synthase activation, endothelium permeability, expression of proinflammatory cytokines, oxidative stress, and aortic collagen 1 deposition, supporting the notion that αEnNaC subunit activation contributes to these vascular responses.
Keywords: aldosterone; cardiovascular disease; endothelium; epithelial sodium channel; inflammation.
Figures





References
-
- Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T American Heart Association Council on H. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension. 2015;66:698–722. - PMC - PubMed
-
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–27. - PubMed
-
- Barhoumi T, Fraulob-Aquino JC, Mian MOR, Ouerd S, Idris-Khodja N, Huo KG, Rehman A, Caillon A, Dancose-Giambattisto B, Ebrahimian T, Lehoux S, Paradis P, Schiffrin EL. Matrix metalloproteinase-2 knockout prevents angiotensin II-induced vascular injury. Cardiovasc Res. 2017;113:1753–1762. - PMC - PubMed
-
- Nguyen Dinh Cat A, Callera GE, Friederich-Persson M, Sanchez A, Dulak-Lis MG, Tsiropoulou S, Montezano AC, He Y, Briones AM, Jaisser F, Touyz RM. Vascular dysfunction in obese diabetic db/db mice involves the interplay between aldosterone/mineralocorticoid receptor and Rho kinase signaling. Sci Rep. 2018;8:2952. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

