Type 2/Th2-driven inflammation impairs olfactory sensory neurogenesis in mouse chronic rhinosinusitis model
- PMID: 29987849
- PMCID: PMC6590422
- DOI: 10.1111/all.13559
Type 2/Th2-driven inflammation impairs olfactory sensory neurogenesis in mouse chronic rhinosinusitis model
Abstract
Background: Chronic rhinosinusitis (CRS) with nasal polyps (CRSwNP) is a chronic inflammatory disease often accompanied by impairment of sense of smell. This symptom has been somewhat overlooked, and its relationship to inflammatory cytokines, tissue compression, neuronal loss, and neurogenesis is still unclear.
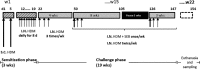
Methods: In order to elucidate potential mechanisms leading to CRS in humans, we have established a type 2/T helper type 2 cell (Th2)-mediated allergic CRS mouse model, based on house dust mite (HDM) and Staphylococcus aureus enterotoxin B (SEB) sensitization. The inflammatory status of the olfactory epithelium (OE) was assessed using histology, biochemistry, and transcriptomics. The sense of smell was evaluated by studying olfactory behavior and recording electro-olfactograms (EOGs).
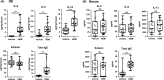
Results: After 22 weeks, a typical type 2/Th2-mediated inflammatory profile was obtained, as demonstrated by increased interleukin (IL)-4, IL-5, and IL-13 in the OE. The number of mast cells and eosinophils was increased, and infiltration of these cells into the olfactory mucosa was also observed. In parallel, transcriptomic and histology analyses indicated a decreased number of immature olfactory neurons, possibly due to decreased renewal. However, the number of mature sensory neurons was not affected and neither the EOG nor olfactory behavior was impaired.
Conclusion: Our mouse model of CRS displayed an allergic response to HDM + SEB administration, including the type 2/Th2 inflammatory profile characteristic of human eosinophilic CRSwNP. Although the sense of smell did not appear to be altered in these conditions, the data reveal the influence of chronic inflammation on olfactory neurogenesis, suggesting that factors unique to humans may be involved in CRSwNP-associated anosmia.
Keywords: Type 2/Th2 inflammation; chronic rhinosinusitis; olfaction; olfactory epithelium; sensory neurogenesis.
© 2018 The Authors. Allergy Published by John Wiley & Sons Ltd.
Conflict of interest statement
Biton B, Bock M‐D, Classe M, Clément M, Didier M, Fourgous V, Françon D, Gorski R, Guillemot J‐C, Haddad E‐B, Le‐Guern J, Leonetti M, Mikol V, Orsini C, Paul P, Ponsolles C, Remaury A, Roche S, Rocheteau‐Beaujouan L, Rouyar A are Sanofi employees and may hold stock and/or stock options in the company.
Figures



References
-
- Hastan D, Fokkens WJ, Bachert C, et al. Chronic rhinosinusitis in Europe–an underestimated disease. A GA(2)LEN study. Allergy. 2011;66:1216‐1223. - PubMed
-
- Fokkens WJ, van Drunen C, Georgalas C, Ebbens F. Role of fungi in pathogenesis of chronic rhinosinusitis: the hypothesis rejected. Curr Opin Otolaryngol Head Neck Surg. 2012;20:19‐23. - PubMed
-
- Bachert C, Claeys SEM, Tomassen P, van Zele T, Zhang N. Rhinosinusitis and asthma: a link for asthma severity. Curr Allergy Asthma Rep. 2010;10:194‐201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

