Second signals rescue B cells from activation-induced mitochondrial dysfunction and death
- PMID: 29988090
- PMCID: PMC6202187
- DOI: 10.1038/s41590-018-0156-5
Second signals rescue B cells from activation-induced mitochondrial dysfunction and death
Abstract
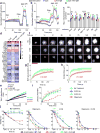
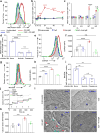
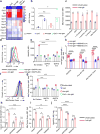
B cells are activated by two temporally distinct signals, the first provided by the binding of antigen to the B cell antigen receptor (BCR), and the second provided by helper T cells. Here we found that B cells responded to antigen by rapidly increasing their metabolic activity, including both oxidative phosphorylation and glycolysis. In the absence of a second signal, B cells progressively lost mitochondrial function and glycolytic capacity, which led to apoptosis. Mitochondrial dysfunction was a result of the gradual accumulation of intracellular calcium through calcium response-activated calcium channels that, for approximately 9 h after the binding of B cell antigens, was preventable by either helper T cells or signaling via the receptor TLR9. Thus, BCR signaling seems to activate a metabolic program that imposes a limited time frame during which B cells either receive a second signal and survive or are eliminated.
Conflict of interest statement
COMPETING INTERESTS STATEMENT.
Authors declare no competing financial interests.
Figures





Comment in
-
B cells race the clock to get a second wind.Nat Immunol. 2018 Aug;19(8):791-793. doi: 10.1038/s41590-018-0166-3. Nat Immunol. 2018. PMID: 29988092 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

