Randomised phase II trial to investigate catumaxomab (anti-EpCAM × anti-CD3) for treatment of peritoneal carcinomatosis in patients with gastric cancer
- PMID: 29988111
- PMCID: PMC6070920
- DOI: 10.1038/s41416-018-0150-6
Randomised phase II trial to investigate catumaxomab (anti-EpCAM × anti-CD3) for treatment of peritoneal carcinomatosis in patients with gastric cancer
Abstract
Background: Peritoneal carcinomatosis (PC) represents an unfavourable prognostic factor for patients with gastric cancer (GC). Intraperitoneal treatment with the bispecific and trifunctional antibody catumaxomab (EpCAM, CD3), in addition to systemic chemotherapy, could improve elimination of PC.
Methods: This prospective, randomised, phase II study investigated the efficacy of catumaxomab followed by chemotherapy (arm A, 5-fluorouracil, leucovorin, oxaliplatin, docetaxel, FLOT) or FLOT alone (arm B) in patients with GC and PC. Primary endpoint was the rate of macroscopic complete remission (mCR) of PC at the time of second diagnostic laparoscopy/laparotomy prior to optional surgery.
Results: Median follow-up was 52 months. Out of 35 patients screened, 15 were allocated to arm A and 16 to arm B. mCR rate was 27% in arm A and 19% in arm B (p = 0.69). Severe side effects associated with catumaxomab were nausea, infection, abdominal pain, and elevated liver enzymes. Median progression-free (6.7 vs. 5.4 months, p = 0.71) and overall survival (13.2 vs. 13.0 months, p = 0.97) were not significantly different in both treatment arms.
Conclusions: Addition of catumaxomab to systemic chemotherapy was feasible and tolerable in advanced GC. Although the primary endpoint could not be demonstrated, results are promising for future investigations integrating intraperitoneal immunotherapy into a multimodal treatment strategy.
Conflict of interest statement
F.L. has received consulting fees from Astellas, BioNTech, Bristol-Myers-Squibb, Eli Lilly, Ganymed Pharmaceuticals, Merck Sharp & Dohme; lecture honoraria from Amgen, Astra Zeneca, Eli Lilly, Merck Sharp & Dohme, Servier and research support from Fresenius Biotech. V.K. has received research support from Fresenius Biotech. S.I. has received consulting fees from AIO gGmbH. SEAB has an advisory role with Merck, Roche, Celgene, Lilly, and Nordic Pharma; S.I. is a speaker for Merck, Roche, Celgene, Lilly, and Nordic Pharma; and has received research grants from Sanofi, Merck, Roche, Celgene, Vifor, Medac, Hospira, and Lilly. H.L. is the CEO of Trion Research and inventor or coinventor of several trifunctional antibody patents. All other authors declare no competing interest.
Figures
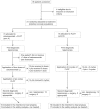

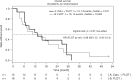
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

