Primary Immunodeficiencies Unravel the Role of IL-2/CD25/STAT5b in Human Natural Killer Cell Maturation
- PMID: 29988287
- PMCID: PMC6023967
- DOI: 10.3389/fimmu.2018.01429
Primary Immunodeficiencies Unravel the Role of IL-2/CD25/STAT5b in Human Natural Killer Cell Maturation
Abstract
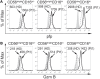
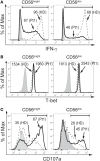
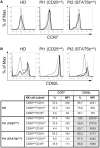
Natural killer (NK) cells play a pivotal role during immunity against viruses and circumstantial evidence also indicates that they can protect the host against developing tumors. Peripheral blood NK cells comprise CD56brightCD16lo/- cells that constitutively express CD25 (IL-2Rα) and CD56dimCD16hi cells that express CD25 upon activation. Using NK cells from two patients, one with a primary immunodeficiency characterized by a homozygous mutation in CD25 (born in year 2007 and studied since she was 3 years old) and one with a homozygous mutation in STAT5b (born in year 1992 and studied since she was 10 years old), we observed that the absence of IL-2 signaling through CD25 promotes the accumulation of CD56brightCD16high NK cells, and that CD56brightCD16lo, CD56brightCD16high, and CD56dimCD16high NK cells of this patient exhibited higher content of perforin and granzyme B, and proliferation capacity, compared to healthy donors. Also, CD56bright and CD56dim NK cells of this patient exhibited a reduced IFN-γ production in response to cytokine stimulation and increased degranulation against K562 cells. Also, the CD25-deficient patient presented a lower frequency of terminally differentiated NK cells in the CD56dimCD16hi NK subpopulation compared to the HD (assessed by CD57 and CD94 expression). Remarkably, CD56dimCD16high NK cells from both patients exhibited notoriously higher expression of CD62L compared to HD, suggesting that in the absence of IL-2 signaling through CD25 and STAT5b, NK cells fail to properly downregulate CD62L during their transition from CD56brightCD16lo/- to CD56dimCD16hi cells. Thus, we provide the first demonstration about the in vivo requirement of the integrity of the IL-2/CD25/STAT5b axis for proper human NK cell maturation.
Keywords: CD25; IL-2; STAT5b; natural killer cells; primary immunodeficiencies.
Figures

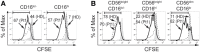

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

