ClC-2 knockdown prevents cerebrovascular remodeling via inhibition of the Wnt/β-catenin signaling pathway
- PMID: 29988306
- PMCID: PMC6022329
- DOI: 10.1186/s11658-018-0095-z
ClC-2 knockdown prevents cerebrovascular remodeling via inhibition of the Wnt/β-catenin signaling pathway
Erratum in
-
Correction: ClC-2 knockdown prevents cerebrovascular remodeling via inhibition of the Wnt/β-catenin signaling pathway.Cell Mol Biol Lett. 2024 Jan 3;29(1):1. doi: 10.1186/s11658-023-00527-9. Cell Mol Biol Lett. 2024. PMID: 38172672 Free PMC article. No abstract available.
Abstract
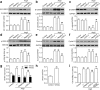
Background: Mishandling of intracellular chloride (Cl-) concentration ([Cl-]i) in cerebrovascular smooth muscle cells is implicated in several pathological processes, including hyperplasia and remodeling. We investigated the effects of ClC-2-mediated Cl- efflux on the proliferation of human brain vascular smooth muscle cells (HBVSMCs) induced by angiotensin II (AngII).
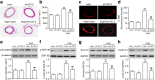
Methods: Cell proliferation and motility were determined using the CCK-8, bromodeoxyuridine staining, wound healing and invasion assays. ClC-2, PCNA, Ki67, survivin and cyclin D1 expression, and β-catenin and GSK-3β phosphorylation were examined using western blotting. Histological analyses were performed using hematoxylin and eosin staining and α-SMA staining.
Results: Our results showed that AngII-induced HBVSMC proliferation was accompanied by a decrease in [Cl-]i and an increase in ClC-2 expression. Inhibition of ClC-2 by siRNA prevented AngII from inducing the efflux of Cl-. AngII-induced HBVSMC proliferation, migration and invasion were significantly attenuated by ClC-2 downregulation. The inhibitory effects of ClC-2 knockout on HBVSMC proliferation and motility were associated with inactivation of the Wnt/β-catenin signaling pathway, as evidenced by inhibition of β-catenin phosphorylation and nuclear translocation, and decrease of GSK-3β phosphorylation and survivin and cyclin D1 expression. Recombinant Wnt3a treatment markedly reversed the effect of ClC-2 knockdown on HBVSMC viability. An in vivo study revealed that knockdown of ClC-2 with shRNA adenovirus ameliorated basilar artery remodeling by inhibiting Wnt/β-catenin signaling in AngII-treated mice.
Conclusion: This study demonstrates that blocking ClC-2-mediated Cl- efflux inhibits AngII-induced cerebrovascular smooth muscle cell proliferation and migration by inhibiting the Wnt/β-catenin pathway. Our data indicate that downregulation of ClC-2 may be a viable strategy in the prevention of hyperplasia and remodeling of cerebrovascular smooth muscle cells.
Keywords: Angiotensin II; Cerebrovascular smooth muscle cells; Chloride; ClC-2; Proliferation; Wnt/β-catenin signaling.
Conflict of interest statement
All animal procedures were in accordance with the institutional guidelines of the Henan People’s Hospital and were approved by the Institutional Animal Ethics Committee.This study is approved by all authors for publication.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Inhibition of angiotensin II-induced cerebrovascular smooth muscle cell proliferation by LRRC8A downregulation through suppressing PI3K/AKT activation.Hum Cell. 2019 Jul;32(3):316-325. doi: 10.1007/s13577-019-00260-6. Epub 2019 May 24. Hum Cell. 2019. PMID: 31127489
-
Acetylshikonin attenuates angiotensin II-induced proliferation and motility of human brain smooth muscle cells by inhibiting Wnt/β-catenin signaling.Hum Cell. 2018 Jul;31(3):242-250. doi: 10.1007/s13577-018-0207-0. Epub 2018 Apr 23. Hum Cell. 2018. PMID: 29687375
-
Silence of ClC-3 chloride channel inhibits cell proliferation and the cell cycle via G/S phase arrest in rat basilar arterial smooth muscle cells.Cell Prolif. 2008 Oct;41(5):775-85. doi: 10.1111/j.1365-2184.2008.00551.x. Cell Prolif. 2008. PMID: 18823498 Free PMC article.
-
The ClC-3 chloride channels in cardiovascular disease.Acta Pharmacol Sin. 2011 Jun;32(6):675-84. doi: 10.1038/aps.2011.30. Epub 2011 May 23. Acta Pharmacol Sin. 2011. PMID: 21602838 Free PMC article. Review.
-
Wnt signalling in smooth muscle cells and its role in cardiovascular disorders.Cardiovasc Res. 2012 Jul 15;95(2):233-40. doi: 10.1093/cvr/cvs141. Epub 2012 Apr 4. Cardiovasc Res. 2012. PMID: 22492675 Review.
Cited by
-
Editorial focus: understanding off-target effects as the key to successful RNAi therapy.Cell Mol Biol Lett. 2019 Dec 9;24:69. doi: 10.1186/s11658-019-0196-3. eCollection 2019. Cell Mol Biol Lett. 2019. PMID: 31867046 Free PMC article. Review.
-
From Channels to Canonical Wnt Signaling: A Pathological Perspective.Int J Mol Sci. 2021 Apr 28;22(9):4613. doi: 10.3390/ijms22094613. Int J Mol Sci. 2021. PMID: 33924772 Free PMC article. Review.
-
Correction: ClC-2 knockdown prevents cerebrovascular remodeling via inhibition of the Wnt/β-catenin signaling pathway.Cell Mol Biol Lett. 2024 Jan 3;29(1):1. doi: 10.1186/s11658-023-00527-9. Cell Mol Biol Lett. 2024. PMID: 38172672 Free PMC article. No abstract available.
-
Inhibition of angiotensin II-induced cerebrovascular smooth muscle cell proliferation by LRRC8A downregulation through suppressing PI3K/AKT activation.Hum Cell. 2019 Jul;32(3):316-325. doi: 10.1007/s13577-019-00260-6. Epub 2019 May 24. Hum Cell. 2019. PMID: 31127489
-
Molecular mechanisms, physiological roles, and therapeutic implications of ion fluxes in bone cells: Emphasis on the cation-Cl- cotransporters.J Cell Physiol. 2022 Dec;237(12):4356-4368. doi: 10.1002/jcp.30879. Epub 2022 Sep 20. J Cell Physiol. 2022. PMID: 36125923 Free PMC article. Review.
References
-
- Bihl JC, Zhang C, Zhao Y, Xiao X, Ma X, Chen Y, Chen S, Zhao B, Chen Y. Angiotensin-(1-7) counteracts the effects of Ang II on vascular smooth muscle cells, vascular remodeling and hemorrhagic stroke: role of the NFsmall ka, CyrillicB inflammatory pathway. Vasc Pharmacol. 2015;73:115–123. doi: 10.1016/j.vph.2015.08.007. - DOI - PMC - PubMed
-
- Ma MM, Lin CX, Liu CZ, Gao M, Sun L, Tang YB, Zhou JG, Wang GL, Guan YY. Threonine532 phosphorylation in ClC-3 channels is required for angiotensin II-induced Cl(−) current and migration in cultured vascular smooth muscle cells. Br J Pharmacol. 2016;173:529–544. doi: 10.1111/bph.13385. - DOI - PMC - PubMed
-
- Galatioto J, Caescu CI, Hansen J, Cook JR, Miramontes I, Iyengar R, Ramirez F. Cell type-specific contributions of the angiotensin II type 1a receptor to aorta homeostasis and aneurysmal disease-brief report. Arterioscler Thromb Vasc Biol. 2018;38:588–591. doi: 10.1161/ATVBAHA.117.310609. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

