Alternative Pathway Is Essential for Glomerular Complement Activation and Proteinuria in a Mouse Model of Membranous Nephropathy
- PMID: 29988342
- PMCID: PMC6023961
- DOI: 10.3389/fimmu.2018.01433
Alternative Pathway Is Essential for Glomerular Complement Activation and Proteinuria in a Mouse Model of Membranous Nephropathy
Abstract
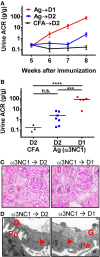
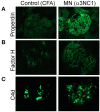
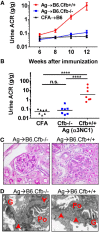
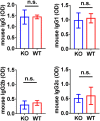
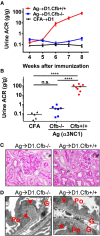
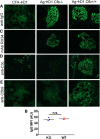
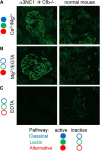
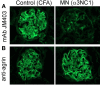
Membranous nephropathy is an immune kidney disease caused by IgG antibodies that form glomerular subepithelial immune complexes. Proteinuria is mediated by complement activation, as a result of podocyte injury by C5b-9, but the role of specific complement pathways is not known. Autoantibodies-mediating primary membranous nephropathy are predominantly of IgG4 subclass, which cannot activate the classical pathway. Histologic evidence from kidney biopsies suggests that the lectin and the alternative pathways may be activated in membranous nephropathy, but the pathogenic relevance of these pathways remains unclear. In this study, we evaluated the role of the alternative pathway in a mouse model of membranous nephropathy. After inducing the formation of subepithelial immune complexes, we found similar glomerular IgG deposition in wild-type mice and in factor B-null mice, which lack a functional alternative pathway. Unlike wild-type mice, mice lacking factor B did not develop albuminuria nor exhibit glomerular deposition of C3c and C5b-9. Albuminuria was also reduced but not completely abolished in C5-deficient mice. Our results provide the first direct evidence that the alternative pathway is necessary for pathogenic complement activation by glomerular subepithelial immune complexes and is, therefore, a key mediator of proteinuria in experimental membranous nephropathy. This knowledge is important for the rational design of new therapies for membranous nephropathy.
Keywords: albuminuria; alternative pathway; complement C5; factor B; glomerulonephritis; membrane attack complex; membranous nephropathy; mouse models.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

