Brain Energy and Oxygen Metabolism: Emerging Role in Normal Function and Disease
- PMID: 29988368
- PMCID: PMC6023993
- DOI: 10.3389/fnmol.2018.00216
Brain Energy and Oxygen Metabolism: Emerging Role in Normal Function and Disease
Abstract
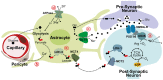
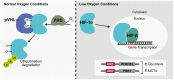
Dynamic metabolic changes occurring in neurons are critically important in directing brain plasticity and cognitive function. In other tissue types, disruptions to metabolism and the resultant changes in cellular oxidative state, such as increased reactive oxygen species (ROS) or induction of hypoxia, are associated with cellular stress. In the brain however, where drastic metabolic shifts occur to support physiological processes, subsequent changes to cellular oxidative state and induction of transcriptional sensors of oxidative stress likely play a significant role in regulating physiological neuronal function. Understanding the role of metabolism and metabolically-regulated genes in neuronal function will be critical in elucidating how cognitive functions are disrupted in pathological conditions where neuronal metabolism is affected. Here, we discuss known mechanisms regulating neuronal metabolism as well as the role of hypoxia and oxidative stress during normal and disrupted neuronal function. We also summarize recent studies implicating a role for metabolism in regulating neuronal plasticity as an emerging neuroscience paradigm.
Keywords: hypoxia; neurodegeneration; neurometabolism; oxidative metabolism; plasticity.
Figures

References
-
- Archer S. L., Huang J. M., Hampl V., Nelson D. P., Shultz P. J., Weir E. K. (1994). Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc. Natl. Acad. Sci. U S A 91, 7583–7587. 10.1073/pnas.91.16.7583 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

