GlnR-Mediated Regulation of Short-Chain Fatty Acid Assimilation in Mycobacterium smegmatis
- PMID: 29988377
- PMCID: PMC6023979
- DOI: 10.3389/fmicb.2018.01311
GlnR-Mediated Regulation of Short-Chain Fatty Acid Assimilation in Mycobacterium smegmatis
Abstract
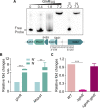
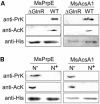
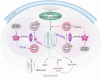
Assimilation of short-chain fatty acids (SCFAs) plays an important role in the survival and lipid biosynthesis of Mycobacteria. However, regulation of this process has not been thoroughly described. In the present work, we demonstrate that GlnR as a well-known nitrogen-sensing regulator transcriptionally modulates the AMP-forming propionyl-CoA synthetase (MsPrpE), and acetyl-CoA synthetases (MsAcs) is associated with SCFAs assimilation in Mycobacterium smegmatis, a model Mycobacterium. GlnR can directly activate the expression of MsprpE and Msacs by binding to their promoter regions based upon sensed nitrogen starvation in the host. Moreover, GlnR can activate the expression of lysine acetyltransferase encoding Mspat, which significantly decreases the activity of MsPrpE and MsAcs through increased acylation. Next, growth curves and resazurin assay show that GlnR can further regulate the growth of M. smegmatis on different SCFAs to control the viability. These results demonstrate that GlnR-mediated regulation of SCFA assimilation in response to the change of nitrogen signal serves to control the survival of M. smegmatis. These findings provide insights into the survival and nutrient utilization mechanisms of Mycobacteria in their host, which may enable new strategies in drug discovery for the control of tuberculosis.
Keywords: GlnR; acylation; nitrogen metabolism; post-translational modification; propionyl-CoA/acetyl-CoA synthetase; short-chain fatty acid.
Figures




Similar articles
-
The Nitrogen Regulator GlnR Directly Controls Transcription of the prpDBC Operon Involved in Methylcitrate Cycle in Mycobacterium smegmatis.J Bacteriol. 2019 Mar 26;201(8):e00099-19. doi: 10.1128/JB.00099-19. Print 2019 Apr 15. J Bacteriol. 2019. PMID: 30745367 Free PMC article.
-
Transcriptional Self-Regulation of the Master Nitrogen Regulator GlnR in Mycobacteria.J Bacteriol. 2023 Apr 25;205(4):e0047922. doi: 10.1128/jb.00479-22. Epub 2023 Mar 21. J Bacteriol. 2023. PMID: 36943048 Free PMC article.
-
Acetyl-CoA synthetases of Saccharopolyspora erythraea are regulated by the nitrogen response regulator GlnR at both transcriptional and post-translational levels.Mol Microbiol. 2017 Mar;103(5):845-859. doi: 10.1111/mmi.13595. Epub 2017 Jan 23. Mol Microbiol. 2017. PMID: 27987242
-
Modulation of Gene Expression in Actinobacteria by Translational Modification of Transcriptional Factors and Secondary Metabolite Biosynthetic Enzymes.Front Microbiol. 2021 Mar 16;12:630694. doi: 10.3389/fmicb.2021.630694. eCollection 2021. Front Microbiol. 2021. PMID: 33796086 Free PMC article. Review.
-
The Balance Metabolism Safety Net: Integration of Stress Signals by Interacting Transcriptional Factors in Streptomyces and Related Actinobacteria.Front Microbiol. 2020 Jan 22;10:3120. doi: 10.3389/fmicb.2019.03120. eCollection 2019. Front Microbiol. 2020. PMID: 32038560 Free PMC article. Review.
Cited by
-
GlnR Negatively Regulates Glutamate-Dependent Acid Resistance in Lactobacillus brevis.Appl Environ Microbiol. 2020 Mar 18;86(7):e02615-19. doi: 10.1128/AEM.02615-19. Print 2020 Mar 18. Appl Environ Microbiol. 2020. PMID: 31953336 Free PMC article.
-
Protein Acetyltransferases Mediate Bacterial Adaptation to a Diverse Environment.J Bacteriol. 2021 Sep 8;203(19):e0023121. doi: 10.1128/JB.00231-21. Epub 2021 Sep 8. J Bacteriol. 2021. PMID: 34251868 Free PMC article. Review.
-
Negative regulation of the acsA1 gene encoding the major acetyl-CoA synthetase by cAMP receptor protein in Mycobacterium smegmatis.J Microbiol. 2022 Dec;60(12):1139-1152. doi: 10.1007/s12275-022-2347-x. Epub 2022 Oct 24. J Microbiol. 2022. PMID: 36279104
-
Factors affecting dysbiosis of the gut microbiota in the elderly and the progress of interventions in traditional Chinese and Western medicine.Front Cell Infect Microbiol. 2025 Mar 24;15:1529347. doi: 10.3389/fcimb.2025.1529347. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40196043 Free PMC article.
-
Dynamic Characterization of Protein and Posttranslational Modification Levels in Mycobacterial Cholesterol Catabolism.mSystems. 2020 Jan 7;5(1):e00424-19. doi: 10.1128/mSystems.00424-19. mSystems. 2020. PMID: 31911463 Free PMC article.
References
-
- Amon J., Bräu T., Grimrath A., Hänssler E., Hasselt K., Höller M., et al. (2008). Nitrogen control in Mycobacterium smegmatis: nitrogen-dependent expression of ammonium transport and assimilation proteins depends on the OmpR-type regulator GlnR. J. Bacteriol. 190 7108–7116. 10.1128/JB.00855-08 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

