Zika Virus Non-structural Protein 4A Blocks the RLR-MAVS Signaling
- PMID: 29988497
- PMCID: PMC6026624
- DOI: 10.3389/fmicb.2018.01350
Zika Virus Non-structural Protein 4A Blocks the RLR-MAVS Signaling
Abstract
Flaviviruses have evolved complex mechanisms to evade the mammalian host immune systems including the RIG-I (retinoic acid-inducible gene I) like receptor (RLR) signaling. Zika virus (ZIKV) is a re-emerging flavivirus that is associated with severe neonatal microcephaly and adult Guillain-Barre syndrome. However, the molecular mechanisms underlying ZIKV pathogenesis remain poorly defined. Here we report that ZIKV non-structural protein 4A (NS4A) impairs the RLR-mitochondrial antiviral-signaling protein (MAVS) interaction and subsequent induction of antiviral immune responses. In human trophoblasts, both RIG-I and melanoma differentiation-associated protein 5 (MDA5) contribute to type I interferon (IFN) induction and control ZIKV replication. Type I IFN induction by ZIKV is almost completely abolished in MAVS-/- cells. NS4A represses RLR-, but not Toll-like receptor-mediated immune responses. NS4A specifically binds the N-terminal caspase activation and recruitment domain (CARD) of MAVS and thus blocks its accessibility by RLRs. Our study provides in-depth understanding of the molecular mechanisms of immune evasion by ZIKV and its pathogenesis.
Keywords: NS4A; RIG-I like receptors; RLR; Zika; flavivirus; non-structural protein 4A.
Figures
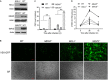

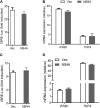
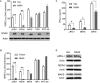
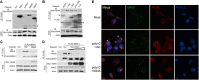
Similar articles
-
Zika Virus Proteins NS2A and NS4A Are Major Antagonists that Reduce IFN-β Promoter Activity Induced by the MDA5/RIG-I Signaling Pathway.J Microbiol Biotechnol. 2019 Oct 28;29(10):1665-1674. doi: 10.4014/jmb.1909.09017. J Microbiol Biotechnol. 2019. PMID: 31581385
-
Flavivirus prM interacts with MDA5 and MAVS to inhibit RLR antiviral signaling.Cell Biosci. 2023 Jan 13;13(1):9. doi: 10.1186/s13578-023-00957-0. Cell Biosci. 2023. PMID: 36639652 Free PMC article.
-
Zika virus antagonizes interferon response in patients and disrupts RIG-I-MAVS interaction through its CARD-TM domains.Cell Biosci. 2019 Jun 7;9:46. doi: 10.1186/s13578-019-0308-9. eCollection 2019. Cell Biosci. 2019. PMID: 31183075 Free PMC article.
-
Accessory Factors of Cytoplasmic Viral RNA Sensors Required for Antiviral Innate Immune Response.Front Immunol. 2016 May 25;7:200. doi: 10.3389/fimmu.2016.00200. eCollection 2016. Front Immunol. 2016. PMID: 27252702 Free PMC article. Review.
-
Mechanisms and pathways of innate immune activation and regulation in health and cancer.Hum Vaccin Immunother. 2014;10(11):3270-85. doi: 10.4161/21645515.2014.979640. Hum Vaccin Immunother. 2014. PMID: 25625930 Free PMC article. Review.
Cited by
-
H1N1 Influenza A Virus Protein NS2 Inhibits Innate Immune Response by Targeting IRF7.Viruses. 2022 Oct 31;14(11):2411. doi: 10.3390/v14112411. Viruses. 2022. PMID: 36366509 Free PMC article.
-
UBX Domain Protein 6 Positively Regulates JAK-STAT1/2 Signaling.J Immunol. 2021 Jun 1;206(11):2682-2691. doi: 10.4049/jimmunol.1901337. Epub 2021 May 21. J Immunol. 2021. PMID: 34021047 Free PMC article.
-
Immune Evasion Strategies Used by Zika Virus to Infect the Fetal Eye and Brain.Viral Immunol. 2020 Jan/Feb;33(1):22-37. doi: 10.1089/vim.2019.0082. Epub 2019 Nov 5. Viral Immunol. 2020. PMID: 31687902 Free PMC article. Review.
-
Zika virus modulates mitochondrial dynamics, mitophagy, and mitochondria-derived vesicles to facilitate viral replication in trophoblast cells.Front Immunol. 2023 Sep 14;14:1203645. doi: 10.3389/fimmu.2023.1203645. eCollection 2023. Front Immunol. 2023. PMID: 37781396 Free PMC article.
-
Flaviviridae Nonstructural Proteins: The Role in Molecular Mechanisms of Triggering Inflammation.Viruses. 2022 Aug 18;14(8):1808. doi: 10.3390/v14081808. Viruses. 2022. PMID: 36016430 Free PMC article. Review.
References
-
- Best S. M., Morris K. L., Shannon J. G., Robertson S. J., Mitzel D. N., Park G. S., et al. (2005). Inhibition of interferon-stimulated JAK-STAT signaling by a tick-borne flavivirus and identification of NS5 as an interferon antagonist. J. Virol. 79 12828–12839. 10.1128/JVI.79.20.12828-12839.2005 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

