Anti-Inflammatory MicroRNAs and Their Potential for Inflammatory Diseases Treatment
- PMID: 29988529
- PMCID: PMC6026627
- DOI: 10.3389/fimmu.2018.01377
Anti-Inflammatory MicroRNAs and Their Potential for Inflammatory Diseases Treatment
Abstract
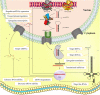
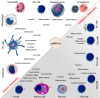
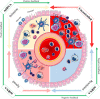
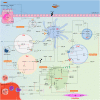
Inflammation is a complicated biological and pathophysiological cascade of responses to infections and injuries, and inflammatory mechanisms are closely related to many diseases. The magnitude, the complicated network of pro- and anti-inflammatory factors, and the direction of the inflammatory response can impact on the development and progression of various disorders. The currently available treatment strategies often target the symptoms and not the causes of inflammatory disease and may often be ineffective. Since the onset and termination of inflammation are crucial to prevent tissue damage, a range of mechanisms has evolved in nature to regulate the process including negative and positive feedback loops. In this regard, microRNAs (miRNAs) have emerged as key gene regulators to control inflammation, and it is speculated that they are fine-tune signaling regulators to allow for proper resolution and prevent uncontrolled progress of inflammatory reactions. In this review, we discuss recent findings related to significant roles of miRNAs in immune regulation, especially the potential utility of these molecules as novel anti-inflammatory agents to treat inflammatory diseases. Furthermore, we discuss the possibilities of using miRNAs as drugs in the form of miRNA mimics or miRNA antagonists.
Keywords: anti-inflammatory microRNA; immune regulation; inflammation; inflammatory diseases; microRNA.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

