Myeloid-Derived Suppressor Cells Induce Podocyte Injury Through Increasing Reactive Oxygen Species in Lupus Nephritis
- PMID: 29988544
- PMCID: PMC6026681
- DOI: 10.3389/fimmu.2018.01443
Myeloid-Derived Suppressor Cells Induce Podocyte Injury Through Increasing Reactive Oxygen Species in Lupus Nephritis
Abstract
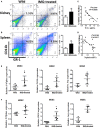
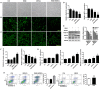
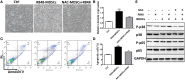
The expansion of myeloid-derived suppressor cells (MDSCs) has been documented in murine models and patients with lupus nephritis (LN), but the exact role of MDSCs in this process remains largely unknown. In this study, we investigated whether MDSCs are involved in the process of podocyte injury in the development of LN. In toll-like receptor-7 (TLR-7) agonist imiquimod-induced lupus mice, we found the severe podocyte injury in glomeruli of lupus mice and significant expansion of MDSCs in spleens and kidneys of lupus mice. The function of TLR-7 activated MDSCs was enhanced including the increased generation of reactive oxygen species (ROS) in vivo and in vitro. Moreover, the ROS production of MDSCs induced podocyte injury through activating the p-38MAPK and NF-kB signaling. Furthermore, we verified that podocyte injury was indeed correlated with expansion of MDSCs and their ROS secretion in LN of pristane-induced lupus mice. These findings first indicate that the podocyte injury in LN was associated with the increased MDSCs in kidney and MDSCs may be a promising therapeutic target of LN in the future.
Keywords: lupus nephritis; myeloid-derived suppressor cells; podocytes; reactive oxygen species; toll-like receptor-7.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

