Regenerative Medicine and Immunomodulatory Therapy: Insights From the Kidney, Heart, Brain, and Lung
- PMID: 29989023
- PMCID: PMC6035130
- DOI: 10.1016/j.ekir.2017.12.012
Regenerative Medicine and Immunomodulatory Therapy: Insights From the Kidney, Heart, Brain, and Lung
Abstract
Regenerative medicine was initially focused on tissue engineering to replace damaged tissues and organs with constructs derived from cells and biomaterials. More recently, this field of inquiry has expanded into exciting areas of translational medicine modulating the body's own endogenous processes, to prevent tissue damage in organs and to repair and regenerate these damaged tissues. This review will focus on recent insights derived from studies in which the manipulation of the innate immunologic system may diminish acute kidney injury and enhance renal repair and recovery without the progression to chronic kidney disease and renal failure. The manner in which these interventions may improve acute and chronic organ dysfunction, including the heart, brain, and lung, will also be reviewed.
Keywords: acute kidney injury; medical device; selective cytopheretic device; translational medicine.
Figures
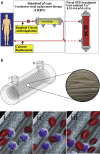

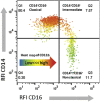
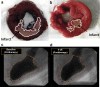
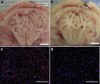
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

