Oncograms Visualize Factors Influencing Long-Term Survival of Cancer Patients Treated with Adenoviral Oncolytic Immunotherapy
- PMID: 29989063
- PMCID: PMC6035494
- DOI: 10.1016/j.omto.2018.04.003
Oncograms Visualize Factors Influencing Long-Term Survival of Cancer Patients Treated with Adenoviral Oncolytic Immunotherapy
Abstract
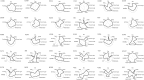
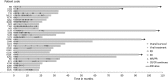
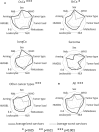
The first US Food and Drug Administration (FDA)- and EMA-approved oncolytic virus has been available since 2015. However, there are no markers available that would predict benefit for the individual patient. During 2007-2012, we treated 290 patients with advanced chemotherapy-refractory cancers, using 10 different oncolytic adenoviruses. Treatments were given in a Finnish Medicines Agency (FIMEA)-regulated individualized patient treatment program (the Advanced Therapy Access Program [ATAP]), which required long-term follow-up of patients, which is presented here. Focusing on the longest surviving patients, some key clinical and biological features are presented as "oncograms." Some key attributes that could be captured in the oncogram are suggested to predict treatment response and survival after oncolytic adenovirus treatment. The oncogram includes immunological laboratory parameters assessed in peripheral blood (leukocytes, neutrophil-to-lymphocyte ratio, interleukin-8 [IL-8], HMGB1, anti-viral neutralizing antibody status), features of the patient (gender, performance status), tumor features (histological tumor type, tumor load, region of metastases), and oncolytic virus-specific features (arming of the virus). The retrospective approach used here facilitates verification in a prospective controlled trial setting. To our knowledge, the oncogram is the first holistic attempt to identify the patients most likely to benefit from adenoviral oncolytic virotherapy.
Keywords: adenovirus; anti-cancer; cancer; immunogram; immunostimulation; immunotherapy; oncogram; oncoimmunology; oncolytic adenovirus; oncolytic virotherapy.
Figures

References
-
- Katz H., Wassie E., Alsharedi M. Checkpoint inhibitors: the new treatment paradigm for urothelial bladder cancer. Med. Oncol. 2017;34:170. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

