Tailor-Made Functional Peptide Self-Assembling Nanostructures
- PMID: 29989255
- PMCID: PMC7616936
- DOI: 10.1002/adma.201707083
Tailor-Made Functional Peptide Self-Assembling Nanostructures
Abstract
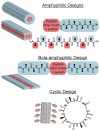
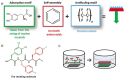
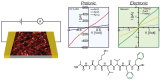
Noncovalent interactions are the main driving force in the folding of proteins into a 3D functional structure. Motivated by the wish to reveal the mechanisms of the associated self-assembly processes, scientists are focusing on studying self-assembly processes of short protein segments (peptides). While this research has led to major advances in the understanding of biological and pathological process, only in recent years has the applicative potential of the resulting self-assembled peptide assemblies started to be explored. Here, major advances in the development of biomimetic supramolecular peptide assemblies as coatings, gels, and as electroactive materials, are highlighted. The guiding lines for the design of helical peptides, β strand peptides, as well as surface binding monolayer-forming peptides that can be utilized for a specific function are highlighted. Examples of their applications in diverse immerging applications in, e.g., ecology, biomedicine, and electronics, are described. Taking into account that, in addition to extraordinary design flexibility, these materials are naturally biocompatible and ecologically friendly, and their production is cost effective, the emergence of devices incorporating these biomimetic materials in the market is envisioned in the near future.
Keywords: bioelectronics; hydrogels; nanostructures; peptides; self-assembly.
© 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

