The lysosomal membrane protein LAMP2A promotes autophagic flux and prevents SNCA-induced Parkinson disease-like symptoms in the Drosophila brain
- PMID: 29989488
- PMCID: PMC6152503
- DOI: 10.1080/15548627.2018.1491489
The lysosomal membrane protein LAMP2A promotes autophagic flux and prevents SNCA-induced Parkinson disease-like symptoms in the Drosophila brain
Abstract
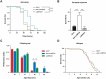
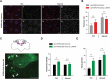
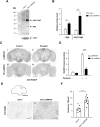
The autophagy-lysosome pathway plays a fundamental role in the clearance of aggregated proteins and protection against cellular stress and neurodegenerative conditions. Alterations in autophagy processes, including macroautophagy and chaperone-mediated autophagy (CMA), have been described in Parkinson disease (PD). CMA is a selective autophagic process that depends on LAMP2A (lysosomal-associated membrane protein 2A), a mammal and bird-specific membrane glycoprotein that translocates cytosolic proteins containing a KFERQ-like peptide motif across the lysosomal membrane. Drosophila reportedly lack CMA and use endosomal microautophagy (eMI) as an alternative selective autophagic process. Here we report that neuronal expression of human LAMP2A protected Drosophila against starvation and oxidative stress, and delayed locomotor decline in aging flies without extending their lifespan. LAMP2A also prevented the progressive locomotor and oxidative defects induced by neuronal expression of PD-associated human SNCA (synuclein alpha) with alanine-to-proline mutation at position 30 (SNCAA30P). Using KFERQ-tagged fluorescent biosensors, we observed that LAMP2A expression stimulated selective autophagy in the adult brain and not in the larval fat body, but did not increase this process under starvation conditions. Noteworthy, we found that neurally expressed LAMP2A markedly upregulated levels of Drosophila Atg5, a key macroautophagy initiation protein, and that it increased the density of Atg8a/LC3-positive puncta, which reflects the formation of autophagosomes. Furthermore, LAMP2A efficiently prevented accumulation of the autophagy defect marker Ref(2)P/p62 in the adult brain under acute oxidative stress. These results indicate that LAMP2A can potentiate autophagic flux in the Drosophila brain, leading to enhanced stress resistance and neuroprotection.
Abbreviations: Act5C: actin 5C; a.E.: after eclosion; Atg5: autophagy-related 5; Atg8a/LC3: autophagy-related 8a; CMA: chaperone-mediated autophagy; DHE: dihydroethidium; elav: embryonic lethal abnormal vision; eMI: endosomal microautophagy; ESCRT: endosomal sorting complexes required for transport; GABARAP: GABA typeA receptor-associated protein; Hsc70-4: heat shock protein cognate 4; HSPA8/Hsc70: heat shock protein family A (Hsp70) member 8; LAMP2: lysosomal associated membrane protein 2; MDA: malondialdehyde; PA-mCherry: photoactivable mCherry; PBS: phosphate-buffered saline; PCR: polymerase chain reaction; PD: Parkinson disease; Ref(2)P/p62: refractory to sigma P; ROS: reactive oxygen species; RpL32/rp49: ribosomal protein L32; RT-PCR: reverse transcription polymerase chain reaction; SING: startle-induced negative geotaxis; SNCA/α-synuclein: synuclein alpha; SQSTM1/p62: sequestosome 1; TBS: Tris-buffered saline; UAS: upstream activating sequence.
Keywords: Autophagy-lysosome pathway; Drosophila melanogaster; Parkinson disease; lysosomal-associated membrane protein 2A (LAMP2A); neuroprotection; synuclein alpha (SNCA).
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
