Hydroamination versus Allylic Amination in Iridium-Catalyzed Reactions of Allylic Acetates with Amines: 1,3-Aminoalcohols via Ester-Directed Regioselectivity
- PMID: 29989803
- PMCID: PMC6264884
- DOI: 10.1021/jacs.8b05683
Hydroamination versus Allylic Amination in Iridium-Catalyzed Reactions of Allylic Acetates with Amines: 1,3-Aminoalcohols via Ester-Directed Regioselectivity
Abstract
In the presence of a neutral dppf-modified iridium catalyst and Cs2CO3, linear allylic acetates react with primary amines to form products of hydroamination with complete 1,3-regioselectivity. The collective data, including deuterium labeling studies, corroborate a catalytic mechanism involving rapid, reversible acetate-directed aminoiridation with inner-sphere/outer-sphere crossover followed by turnover-limiting proto-demetalation mediated by amine.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
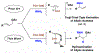

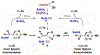
References
-
-
For selected reviews on metal catalyzed hydroamination, see:
- Müller TE; Beller M Chem. Rev 1998. 98 675. - PubMed
- Hong S; Marks TJ ,Acc. Chem. Res 2004, 37 673. - PubMed
- Severin R; Doye S Chem. Soc. Rev 2007. 36 1407. - PubMed
- Müller TE; Hultzsch KC; Yus M; Foubelo F; Tada. M. Chem. Rev 2008. 108 3795. - PubMed
- Hesp KD; Stradiotto M ChemCatChem 2010, 2 1192.
- Schafer LL; Yim JCH; Yonson N In Metal-Catalyzed Cross Coupling Reactions and More; De Meijere A, Bräse S, Oestreich M, Eds.; Wiley-VCH: Weinheim, Germany, 2014; Vol. 3, p 1135.
- Huang L; Arndt M; Gooβen K; Heydt H; Gooβen L J. Chem. Rev 2015. 115 2596. - PubMed
- Gupta AK; Hull KL Synlett 2015. 26 1779.
-
-
-
For related photoredox-mediated olefin hydroaminations, see:
- Musacchio AJ; Nguyen LQ; Beard GH; Knowles RR J. Am. Chem. Soc 2014, 136, 12217. - PubMed
- Miller DC; Choi GJ; Orbe HS; Knowles RR J. Am. Chem. Soc 2015, 137, 13492. - PMC - PubMed
- Musacchio AJ; Lainhart BC; Zhang X; Naguib SG; Sherwood TC; Knowles RR Science 2017, 355 727. - PMC - PubMed
-
-
-
For intermolecular rhodium catalyzed olefin hydroamination, see:
- Coulson DR Tetrahedron Lett 1971, 5 429.
- Brunet J-J; Neibecker D; Philippot K J. Chem. Soc. Chem. Commun 1992, 1215.
- Beller M; Eichberger M; Trauthwein H Angew. Chem. Int. Ed. Engl. 1997, 36, 2225.
- Beller M; Trauthwein H; Eichberger M; Breindl C; Herwig J; Müller TE; Thiel OR Chem. Eur. J 1999, 5, 1306.
- Beller M; Trauthwein H; Eichberger M; Breindl C; Müller TE Eur. J. Inorg. Chem 1999, 1121.
- Utsunomiya M; Kuwano R; Kawatsura M; Hartwig JF J. Am. Chem. Soc 2003, 125, 5608. - PubMed
- Baudequin C; Brunet J-J; Rodriguez-Zubiri M Organometallics 2007, 26, 5264.
- Jiménez MV; Pérez-Torrente JJ; Bartolomé MI; Lahoz FJ; Oro LA Chem. Commun 2010, 46, 5322. - PubMed
- Béthegnies A; Kirkina VA; Filippov OA; Daran JC; Belkova NV; Shubina E; Poli R Inorg. Chem 2011, 50, 12539. - PubMed
- Ickes AR; Ensign SC; Gupta AK; Hull KL J. Am. Chem. Soc 2014, 136, 11256. - PubMed
- Ensign SC; Vanable EP; Kortman GD; Weir LJ; Hull KL J. Am. Chem. Soc 2015, 137, 13748. - PubMed
-
-
-
For intramolecular rhodium catalyzed olefin hydroamination, see:
- Diamond SE; Szalkiewicz A; Mares F J. Am. Chem. Soc 1979, 101, 490.
- Takemiya A; Hartwig JF J. Am. Chem. Soc 2006, 128, 6042. - PubMed
- Liu Z; Hartwig JF J. Am. Chem. Soc 2008, 130, 1570. - PMC - PubMed
- Bauer EB; Andavan GTS; Hollis TK; Rubio RJ; Cho J; Kuchenbeiser GR; Helgert TR; Letko CS; Tham FS Org. Lett 2008, 10, 1175. - PubMed
- Shen X; Buchwald SL Angew. Chem. Int. Ed 2010, 49, 564. - PMC - PubMed
- Julian LD; Hartwig JF J. Am. Chem. Soc 2010, 132, 13813. - PMC - PubMed
- Liu Z; Yamamichi H; Madrahimov ST; Hartwig JF J. Am. Chem. Soc 2011, 133, 2772. - PMC - PubMed
- Hua C; Vuong KQ; Bhadbhade M; Messerle BA Organometallics 2012, 31, 1790.
-
-
-
For intermolecular iridium catalyzed olefin hydroamination, see:
- Casalnuovo AL; Calabrese JC; Milstein D J. Am. Chem. Soc 1988, 110, 6738.
- Dorta R; Egli P; Zurcher F; Togni A J. Am. Chem. Soc 1997, 119, 10857.
- Zhou J; Hartwig JF J. Am. Chem. Soc 2008, 130, 12220. - PubMed
- Sevov CS; Zhou J; Hartwig JF J. Am. Chem. Soc 2012, 134, 11960. - PubMed
- Pan S; Endo K; Shibata T Org. Lett 2012, 14, 780. - PubMed
- Sevov CS; Zhou J; Hartwig JF J. Am. Chem. Soc 2014, 136, 3200. - PubMed
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

