Patient-Reported Toxicity During Pelvic Intensity-Modulated Radiation Therapy: NRG Oncology-RTOG 1203
- PMID: 29989857
- PMCID: PMC6097832
- DOI: 10.1200/JCO.2017.77.4273
Patient-Reported Toxicity During Pelvic Intensity-Modulated Radiation Therapy: NRG Oncology-RTOG 1203
Erratum in
Abstract
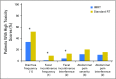
Purpose NRG Oncology/RTOG 1203 was designed to compare patient-reported acute toxicity and health-related quality of life during treatment with standard pelvic radiation or intensity-modulated radiation therapy (IMRT) in women with cervical and endometrial cancer. Methods Patients were randomly assigned to standard four-field radiation therapy (RT) or IMRT radiation treatment. The primary end point was change in patient-reported acute GI toxicity from baseline to the end of RT, measured with the bowel domain of the Expanded Prostate Cancer Index Composite (EPIC). Secondary end points included change in patient-reported urinary toxicity, change in GI toxicity measured with the Patient-Reported Outcome Common Terminology Criteria for Adverse Events, and quality of life measured with the Trial Outcome Index. Results From 2012 to 2015, 289 patients were enrolled, of whom 278 were eligible. Between baseline and end of RT, the mean EPIC bowel score declined 23.6 points in the standard RT group and 18.6 points in the IMRT group ( P = .048), the mean EPIC urinary score declined 10.4 points in the standard RT group and 5.6 points in the IMRT group ( P = .03), and the mean Trial Outcome Index score declined 12.8 points in the standard RT group and 8.8 points in the IMRT group ( P = .06). At the end of RT, 51.9% of women who received standard RT and 33.7% who received IMRT reported frequent or almost constant diarrhea ( P = .01), and more patients who received standard RT were taking antidiarrheal medications four or more times daily (20.4% v 7.8%; P = .04). Conclusion Pelvic IMRT was associated with significantly less GI and urinary toxicity than standard RT from the patient's perspective.
Trial registration: ClinicalTrials.gov NCT01672892.
Figures

Comment in
-
Scores and Misses With New Technology-Walking the Narrow Path of Evidence.Int J Radiat Oncol Biol Phys. 2019 Oct 1;105(2):237-241. doi: 10.1016/j.ijrobp.2019.05.015. Int J Radiat Oncol Biol Phys. 2019. PMID: 31492378 No abstract available.
References
-
- Noone AM, Howlader N, Krapcho M, et al. (eds): SEER Cancer Statistics Review, 1975-2015, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2015/
-
- Rotman M, Sedlis A, Piedmonte MR, et al. : A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: Follow-up of a gynecologic oncology group study. Int J Radiat Oncol Biol Phys 65:169-176, 2006 - PubMed
-
- Peters WA, III, Liu PY, Barrett RJ, II, et al. : Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 18:1606-1613, 2000 - PubMed
-
- Keys HM, Roberts JA, Brunetto VL, et al. : A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol Oncol 92:744-751, 2004 - PubMed
-
- Nout RA, Putter H, Jürgenliemk-Schulz IM, et al. : Five-year quality of life of endometrial cancer patients treated in the randomised Post Operative Radiation Therapy in Endometrial Cancer (PORTEC-2) trial and comparison with norm data. Eur J Cancer 48:1638-1648, 2012 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

