Caenorhabditis elegans respond to high-glucose diets through a network of stress-responsive transcription factors
- PMID: 29990370
- PMCID: PMC6039004
- DOI: 10.1371/journal.pone.0199888
Caenorhabditis elegans respond to high-glucose diets through a network of stress-responsive transcription factors
Abstract
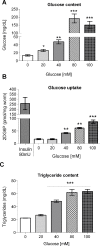
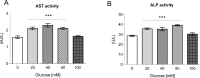
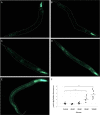
High-glycemic-index diets, as well as a sedentary lifestyle are considered as determinant factors for the development of obesity, type 2 diabetes, and cardiovascular diseases in humans. These diets have been shown to shorten the life span of C. elegans in a manner that is dependent on insulin signaling, but the participation of other signaling pathways have not been addressed. In this study, we have determined that worms fed with high-glucose diets show alterations in glucose content and uptake, triglyceride content, body size, number of eggs laid, egg-laying defects, and signs of oxidative stress and accelerated aging. Additionally, we analyzed the participation of different key regulators of carbohydrate and lipid metabolism, oxidative stress and longevity such as SKN-1/NRF2, HIF-1/HIF1α, SBP-1/SREBP, CRH-1/CREB, CEP-1/p53, and DAF-16/FOXO, in the reduction of lifespan in glucose-fed worms.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


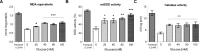
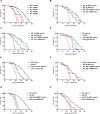
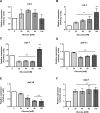
References
-
- Lempradl A, Pospisilik JA, Penninger JM. Exploring the emerging complexity in transcriptional regulation of energy homeostasis. Nat Rev Genet. 2015;16(11):665–681. Epub 2015/10/16. doi: 10.1038/nrg3941 - DOI - PubMed
-
- Mondoux MA, Love DC, Ghosh SK, Fukushige T, Bond M, Weerasinghe GR, et al. O-linked-N-acetylglucosamine cycling and insulin signaling are required for the glucose stress response in Caenorhabditis elegans. Genetics. 2011;188(2):369–382. doi: 10.1534/genetics.111.126490 - DOI - PMC - PubMed
-
- Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24(5):981–990. doi: 10.1016/j.cellsig.2012.01.008 - DOI - PMC - PubMed
-
- Bonomini F, Rodella LF, Rezzani R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015;6(2):109–120. Epub 2015/03/31. doi: 10.14336/AD.2014.0305 - DOI - PMC - PubMed
-
- Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci USA. 2006;103(8):2653–2658. Epub 2006/02/16. doi: 10.1073/pnas.0511154103 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

