Structure-Activity Relationships of the Competence Stimulating Peptide in Streptococcus mutans Reveal Motifs Critical for Membrane Protease SepM Recognition and ComD Receptor Activation
- PMID: 29990430
- PMCID: PMC6138527
- DOI: 10.1021/acsinfecdis.8b00115
Structure-Activity Relationships of the Competence Stimulating Peptide in Streptococcus mutans Reveal Motifs Critical for Membrane Protease SepM Recognition and ComD Receptor Activation
Abstract
Streptococcus mutans ( S. mutans) is a Gram-positive human pathogen that is one of the major contributors to dental caries, a condition with an economic cost of over $100 billion per year in the United States. S. mutans secretes a 21-amino-acid peptide termed the competence stimulating peptide (21-CSP) to assess its population density in a process termed quorum sensing (QS) and to initiate a variety of phenotypes such as biofilm formation and bacteriocin production. 21-CSP is processed by a membrane bound protease SepM into active 18-CSP, which then binds to the ComD receptor. This study seeks to determine the molecular mechanism that ties 21-CSP:SepM recognition and 18-CSP:ComD receptor binding and to identify QS modulators with distinct activity profiles. To this end, we conducted systematic replacement of the amino acid residues in both 21-CSP and 18-CSP and assessed the ability of the mutated analogs to modulate QS. We identified residues that are important to SepM recognition and ComD receptor binding. Our results shed light on the S. mutans competence QS pathway at the molecular level. Moreover, our structural insights of the CSP signal can be used to design QS-based anti-infective therapeutics against S. mutans.
Keywords: Streptococcus mutans; competence stimulating peptide; quorum sensing.
Figures

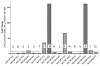
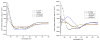

References
-
- Bassler BL. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr Opin Microbiol. 1999;2:582–587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

