Comparison of Antifungal Azole Interactions with Adult Cytochrome P450 3A4 versus Neonatal Cytochrome P450 3A7
- PMID: 29991575
- PMCID: PMC6081698
- DOI: 10.1124/dmd.118.082032
Comparison of Antifungal Azole Interactions with Adult Cytochrome P450 3A4 versus Neonatal Cytochrome P450 3A7
Abstract
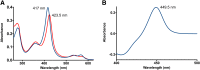
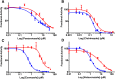
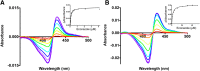
Adult drug metabolism is dominated by cytochrome P450 3A4 (CYP3A4), which is often inhibited by antifungal azole drugs, resulting in potential alterations in drug metabolism and adverse drug/drug interactions. In the fetal and neonatal stages of life, the 87% identical cytochrome P450 3A7 (CYP3A7) is expressed but not CYP3A4. Azole antifungals developed for adults are also used in neonates, assuming they interact similarly with both enzymes, but systematic information is lacking. Herein a method was developed for generating recombinant purified CYP3A7. Thirteen different azoles were then evaluated for binding and inhibition of purified human CYP3A4 versus CYP3A7. All imidazole-containing azoles bound both enzymes via coordination to the heme iron and inhibited both with IC50 values ranging from 180 nM for clotrimazole to the millimolar range for imidazole itself. Across this wide range of potencies, CYP3A4 was consistently inhibited more strongly than CYP3A7, with clotrimazole being the least selective (1.5-fold) inhibitor and econazole the most selective (12-fold). Observations for 1,2,4-triazole-containing azoles were more varied. Most bound to CYP3A4 via coordination to the heme iron, but several also demonstrated evidence of a distinct binding mode at low concentrations. However, only posaconazole inhibited CYP3A4. Of the triazoles, only posaconazole inhibited CYP3A7, again less potently than CYP3A4. Spectral evidence for binding was weak or nonexistent for all triazoles. Overall, although the details of binding interactions do vary, the same azole compounds inhibit both enzymes, albeit with weaker interactions with CYP3A7 compared with CYP3A4.
Copyright © 2018 by The American Society for Pharmacology and Experimental Therapeutics.
Figures

References
-
- Blake MJ, Castro L, Leeder JS, Kearns GL. (2005) Ontogeny of drug metabolizing enzymes in the neonate. Semin Fetal Neonatal Med 10:123–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

