Molecular detection of human papillomavirus (HPV) in highly fragmented DNA from cervical cancer biopsies using double-nested PCR
- PMID: 29992095
- PMCID: PMC6035908
- DOI: 10.1016/j.mex.2018.05.018
Molecular detection of human papillomavirus (HPV) in highly fragmented DNA from cervical cancer biopsies using double-nested PCR
Abstract
Archived Formalin-Fixed Paraffin-Embedded (FFPE) tissue specimens can be a valuable source of human papillomavirus (HPV) nucleic acids for molecular biological analyses in retrospective studies. Although successful amplification with polymerase chain reaction (PCR) is essential for analysis of HPV DNA extracted from cervical FFPE specimens, extensive DNA damage due to cross-linking and fragmentation results in poor yield. Therefore, techniques to improve the diagnostic rate and sensitivity from FFPE tissues through PCR is highly desired and of wider interest. To overcome this, a highly sensitive double-nested PCR methodology was designed and optimized for limited-resource laboratories coupled with an organic extraction of DNA. This method allows the detection of a broad range of HPV genotypes and also allowing the sequencing of the final amplicon. Validation of the new approach developed was done with an automated DNA extraction coupled with Real Time PCR. Results showed that the proposed method achieves 96.3% of HPV detection as compared to 100% Abbott m2000rt used as 'gold standard'. Moreover, the concordance rate between the two methods was equal for detecting HPV -16 or -18 genotypes. Nevertheless, the newly introduced assay has an advantage of: •Simultaneously identifying broad range of HPV genotypes besides HPV-16 and -18 from clinical samples.•It is an easy and cost-effective method that can be beneficial in resource-limited setting and can be employed for various molecular applications.•The method is indicated for highly degraded FFPE samples.
Keywords: DNA; DNA sequencing; Double-nested PCR; Double-nested PCR for detecting and genotyping HPV; FFPE; HPV; RFLP.
Figures

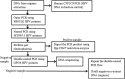
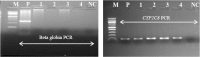

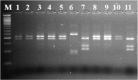
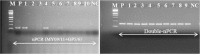
References
-
- Walboomers J.M., Jacobs M.V., Manos M.M., Bosch F.X., Kummer J.A., Shah K.V., Snijders P.J., Peto J., Meijer C.J., Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999;189(1):12–19. - PubMed
-
- de Villiers E.M., Fauquet C., Broker T.R., Bernard H.U., zur Hause H. Classification of papillomaviruses. Virol. J. 2004;324(1):17–27. - PubMed
-
- Smith J.S., Lindsay L., Hoots B., Keys J., Franceschi S., Winer R.G.M., Clifford G.M. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta- analysis update. Int. J. Cancer. 2007;121(3):621–632. - PubMed
-
- Maza M., Gage J.C. Considerations for HPV primary screening in lower-middle income countries. Prev. Med. 2017;98:39–41. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

