GMP Production and Scale-Up of Adherent Neural Stem Cells with a Quantum Cell Expansion System
- PMID: 29992178
- PMCID: PMC6037686
- DOI: 10.1016/j.omtm.2018.05.006
GMP Production and Scale-Up of Adherent Neural Stem Cells with a Quantum Cell Expansion System
Abstract
Cell-based therapies hold great promise for a myriad of clinical applications. However, as these therapies move from phase I to phase II and III trials, there is a need to improve scale-up of adherent cells for the production of larger good manufacturing practice (GMP) cell banks. As we advanced our neural stem cell (NSC)-mediated gene therapy trials for glioma to include dose escalation and multiple treatment cycles, GMP production using cell factories (CellStacks) generated insufficient neural stem cell (NSC) yields. To increase yield, we developed an expansion method using the hollow fiber quantum cell expansion (QCE) system. Seeding of 5.2 × 107 NSCs in a single unit yielded up to 3 × 109 cells within 10 days. These QCE NSCs showed genetic and functional stability equivalent to those expanded by conventional flask-based methods. We then expanded the NSCs in 7 units simultaneously to generate a pooled GMP-grade NSC clinical lot of more than 1.5 × 1010 cells in only 9 days versus 8 × 109 over 6 weeks in CellStacks. We also adenovirally transduced our NSCs within the QCE. We found the QCE system enabled rapid cell expansion and increased yield while maintaining cell properties and reducing process time, labor, and costs with improved efficiency and reproducibility.
Keywords: GMP; HB1.F3.CD; adherent cells; bioreactor; clinical grade; manufacturing; neural stem cells; quantum cell expansion system.
Figures
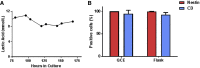


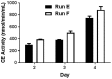
References
-
- Mirfeizi L., Stratton J.A., Kumar R., Shah P., Agabalyan N., Stykel M.G., Midha R., Biernaskie J., Kallos M.S. Serum-free bioprocessing of adult human and rodent skin-derived Schwann cells: implications for cell therapy in nervous system injury. J. Tissue Eng. Regen. Med. 2017;11:3385–3397. - PubMed
-
- Tozetti P.A., Caruso S.R., Mizukami A., Fernandes T.R., da Silva F.B., Traina F., Covas D.T., Orellana M.D., Swiech K. Expansion strategies for human mesenchymal stromal cells culture under xeno-free conditions. Biotechnol. Prog. 2017;33:1358–1367. - PubMed
-
- Lambrechts T., Papantoniou I., Rice B., Schrooten J., Luyten F.P., Aerts J.M. Large-scale progenitor cell expansion for multiple donors in a monitored hollow fibre bioreactor. Cytotherapy. 2016;18:1219–1233. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

