Microphysiological flux balance platform unravels the dynamics of drug induced steatosis
- PMID: 29992215
- PMCID: PMC7004819
- DOI: 10.1039/c8lc00357b
Microphysiological flux balance platform unravels the dynamics of drug induced steatosis
Abstract
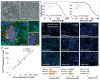
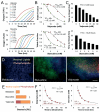
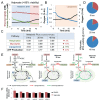
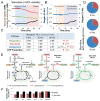
Drug development is currently hampered by the inability of animal experiments to accurately predict human response. While emerging organ on chip technology offers to reduce risk using microfluidic models of human tissues, the technology still mostly relies on end-point assays and biomarker measurements to assess tissue damage resulting in limited mechanistic information and difficulties to detect adverse effects occurring below the threshold of cellular damage. Here we present a sensor-integrated liver on chip array in which oxygen is monitored using two-frequency phase modulation of tissue-embedded microprobes, while glucose, lactate and temperature are measured in real time using microfluidic electrochemical sensors. Our microphysiological platform permits the calculation of dynamic changes in metabolic fluxes around central carbon metabolism, producing a unique metabolic fingerprint of the liver's response to stimuli. Using our platform, we studied the dynamics of human liver response to the epilepsy drug Valproate (Depakine™) and the antiretroviral medication Stavudine (Zerit™). Using E6/E7LOW hepatocytes, we show TC50 of 2.5 and 0.8 mM, respectively, coupled with a significant induction of steatosis in 2D and 3D cultures. Time to onset analysis showed slow progressive damage starting only 15-20 hours post-exposure. However, flux analysis showed a rapid disruption of metabolic homeostasis occurring below the threshold of cellular damage. While Valproate exposure led to a sustained 15% increase in lipogenesis followed by mitochondrial stress, Stavudine exposure showed only a transient increase in lipogenesis suggesting disruption of β-oxidation. Our data demonstrates the importance of tracking metabolic stress as a predictor of clinical outcome.
Conflict of interest statement
Y. N. holds equity in Tissue Dynamics Ltd. and chairs its scientific advisory board.
Figures

References
-
- Olson H, Betton G, Robinson D, Thomas K, Monro A, Kolaja G, Lilly P, Sanders J, Sipes G, Bracken W, Dorato M, et al. Regul Toxicol Pharmacol. 2000;32:56–67. - PubMed
-
- O’Brien PJ, Irwin W, Diaz D, Howard-Cofield E, Krejsa CM, Slaughter MR, Gao B, Kaludercic N, Angeline A, Bernardi P, Brain P, et al. Arch Toxicol. 2006;80:580–604. - PubMed
-
- Bhatia SN, Ingber DE. Nat Biotechnol. 2014;32:760–772. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

