α7 Nicotinic Acetylcholine Receptor Regulates the Function and Viability of L Cells
- PMID: 29992246
- PMCID: PMC6456923
- DOI: 10.1210/en.2018-00433
α7 Nicotinic Acetylcholine Receptor Regulates the Function and Viability of L Cells
Abstract
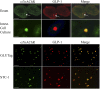
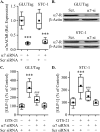
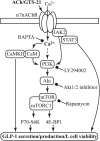
Enteroendocrine L cells secrete the incretin hormone glucagon-like peptide-1 (GLP-1), and they also express the α7 nicotinic acetylcholine receptor (α7nAChR), which may regulate GLP-1 secretion. Here, GTS-21, a selective α7nAChR agonist, was used to examine the effect of α7nAChR activation in L-cell lines, mouse intestinal primary cell cultures, and C57BL/6 mice. GTS-21 stimulated GLP-1 secretion in vitro, and this effect was attenuated by an α7nAChR antagonist or by α7nAChR-specific small interfering RNA. Under in vitro cell culture conditions of glucotoxicity, GTS-21 restored GLP-1 secretion and improved L-cell viability while also acting in vivo to raise levels of circulating GLP-1 in mice. To assess potential signaling mechanisms underlying these actions of GTS-21, we first monitored Ca2+, cAMP, and phosphatidylinositol 3-kinase (PI3K) activity. As expected for a GLP-1 secretagogue promoting Ca2+ influx through α7nAChR cation channels, [Ca2+]i increased in response to GTS-21, but [cAMP]i was unchanged. Surprisingly, pharmacological inhibition of growth factor signaling pathways revealed that GTS-21 also acts on the PI3K-protein kinase B-mammalian target of rapamycin pathway to promote L-cell viability. Moreover, the Ca2+ chelator BAPTA-AM counteracted GTS-21‒stimulated PI3K activity, thereby indicating unexpected crosstalk of L-cell Ca2+ and growth factor signaling pathways. Collectively, these data demonstrate that α7nAChR activation enhances GLP-1 secretion by increasing levels of cytosolic Ca2+ while also revealing Ca2+- and PI3K-dependent processes of α7nAChR activation that promote L-cell survival.
Figures






References
-
- Vilsbøll T, Holst JJ. Incretins, insulin secretion and type 2 diabetes mellitus. Diabetologia. 2004;47(3):357–366. - PubMed
-
- Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044(1):127–131. - PubMed
-
- Krieger JP, Arnold M, Pettersen KG, Lossel P, Langhans W, Lee SJ. Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes. 2016;65(1):34–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

