Electromagnetic interference in cardiac electronic implants caused by novel electrical appliances emitting electromagnetic fields in the intermediate frequency range: a systematic review
- PMID: 29992289
- PMCID: PMC6365808
- DOI: 10.1093/europace/euy155
Electromagnetic interference in cardiac electronic implants caused by novel electrical appliances emitting electromagnetic fields in the intermediate frequency range: a systematic review
Abstract
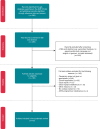
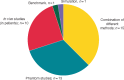
Electromagnetic fields (EMF) in the intermediate frequency (IF) range are generated by many novel electrical appliances, including electric vehicles, radiofrequency identification systems, induction hobs, or energy supply systems, such as wireless charging systems. The aim of this systematic review is to evaluate whether cardiovascular implantable electronic devices (CIEDs) are susceptible to electromagnetic interference (EMI) in the IF range (1 kHz-1 MHz). Additionally, we discuss the advantages and disadvantages of the different types of studies used to investigate EMI. Using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement, we collected and evaluated studies examining EMI in in vivo studies, in vitro studies (phantom studies, benchmark tests), and simulation studies. Our analysis revealed that cardiac implants are susceptible to malfunction induced by EMF in the IF range. Electromagnetic interference may in particular be provoked by security systems and induction hobs. The results of the studies evaluated in this systematic review further indicate that the likelihood for EMI is dependent on exposure-related parameters (field strength, frequency, and modulation) and on implant- as well as on lead-related parameters (model, type of implant, implant sensitivity setting, lead configuration, and implantation site). The review shows that the factors influencing EMI are not sufficiently characterized and EMF limit values for CIED patients cannot be derived yet. Future studies should therefore, consider exposure-related parameters as well as implant- and lead-related parameters systematically. Additionally, worst-case scenarios should be considered in all study types where possible.
Figures
References
-
- Lahor-Soler E, Miranda-Rius J, Brunet-Llobet L, Sabate de la Cruz X.. Capacity of dental equipment to interfere with cardiac implantable electrical devices. Eur J Oral Sci 2015;123:194–201. - PubMed
-
- Mond HG, Proclemer A.. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009—a World Society of Arrhythmia's Project. Pacing Clin Electrophysiol 2011;34:1013–27. - PubMed
-
- Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT. et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol 2011;58:1001–6. - PubMed
-
- Raatikainen MJP, Arnar DO, Merkely B, Nielsen JC, Hindricks G, Heidbuchel H. et al. A decade of information on the use of cardiac implantable electronic devices and interventional electrophysiological procedures in the European Society of Cardiology Countries: 2017 Report from the European Heart Rhythm Association. Europace 2017;19:ii1–90. - PubMed
-
- Gajsek P, Ravazzani P, Wiart J, Grellier J, Samaras T, Thuroczy G.. Electromagnetic field exposure assessment in Europe radiofrequency fields (10 MHz-6 GHz). J Expo Sci Environ Epidemiol 2015;25:37–44. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

