Efficacy and safety of everolimus plus exemestane in postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative locally advanced or metastatic breast cancer: Results of the single-arm, phase IIIB 4EVER trial
- PMID: 29992557
- PMCID: PMC6587781
- DOI: 10.1002/ijc.31738
Efficacy and safety of everolimus plus exemestane in postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative locally advanced or metastatic breast cancer: Results of the single-arm, phase IIIB 4EVER trial
Abstract
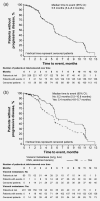
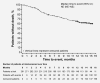
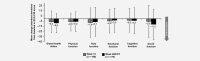
In BOLERO-2, adding everolimus to exemestane resulted in a twofold increase in median progression-free survival (PFS) vs exemestane in postmenopausal women with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer (aBC) after progression on a non-steroidal aromatase inhibitor (NSAI). Here, we report on the open-label, single-arm, phase IIIB 4EVER trial (NCT01626222). This trial evaluated the clinical effectiveness of everolimus plus exemestane in postmenopausal women with HR+, HER2- aBC who had progressed on or after an NSAI, but with no restrictions on the time of progression after NSAI, prior chemotherapy for advanced disease or previous exemestane. The primary endpoint was overall response rate (ORR; i.e. the percentage of patients with a best overall response of complete or partial response per RECIST 1.1) within the first 24 weeks of treatment. Secondary endpoints included PFS, overall survival, safety and health-related quality of life. Between June 2012 and November 2013, 299 patients were enrolled at 82 German centers: 281 patients were evaluable for efficacy and 299 for safety. The ORR was 8.9% (95% confidence interval [CI]: 5.8-12.9%). Median PFS was 5.6 months (95% CI: 5.4-6.0 months). The most frequent grade 3/4 adverse events were stomatitis (8.4%), general physical health deterioration (6.7%), dyspnea (4.7%) and anemia (4.3%). The ORR in 4EVER was lower than in BOLERO-2, likely due to inclusion of patients with more advanced disease and extensive pretreatment. These data confirm the clinical benefits and known safety profile of everolimus plus exemestane in postmenopausal women with HR+, HER2- aBC.
Keywords: HER2-negative; advanced breast cancer; estrogen receptor-positive; everolimus; exemestane.
© 2018 The Authors. International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Figures
References
-
- National Comprehensive Cancer Network, (NCCN) . Breast Cancer. v2, 2017.
-
- Cardoso F, Costa A, Senkus E, et al. 3rd ESO‐ESMO international consensus guidelines for Advanced Breast Cancer (ABC 3). Breast 2017;31:244–259. - PubMed
-
- Rugo HS, Rumble BR, Macrae E, et al. Endocrine therapy for hormone receptor–positive metastatic breast cancer: American Society of Clinical Oncology guideline. J Clin Oncol 2016;34:3069–3103. - PubMed
-
- U.S. Food and Drug Administration . FDA approves abemaciclib for HR‐positive, HER2‐negative breast cancer. Available at: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm578081.htm.
-
- Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor‐positive advanced breast cancer. J Clin Oncol 2010;28:4594–4600. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

