Gypenosides improve diabetic cardiomyopathy by inhibiting ROS-mediated NLRP3 inflammasome activation
- PMID: 29993180
- PMCID: PMC6111804
- DOI: 10.1111/jcmm.13743
Gypenosides improve diabetic cardiomyopathy by inhibiting ROS-mediated NLRP3 inflammasome activation
Abstract
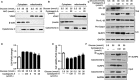
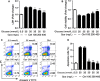
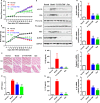
NLRP3 inflammasome activation plays an important role in diabetic cardiomyopathy (DCM), which may relate to excessive production of reactive oxygen species (ROS). Gypenosides (Gps), the major ingredients of Gynostemma pentaphylla (Thunb.) Makino, have exerted the properties of anti-hyperglycaemia and anti-inflammation, but whether Gps improve myocardial damage and the mechanism remains unclear. Here, we found that high glucose (HG) induced myocardial damage by activating the NLRP3 inflammasome and then promoting IL-1β and IL-18 secretion in H9C2 cells and NRVMs. Meanwhile, HG elevated the production of ROS, which was vital to NLRP3 inflammasome activation. Moreover, the ROS activated the NLRP3 inflammasome mainly by cytochrome c influx into the cytoplasm and binding to NLRP3. Inhibition of ROS and cytochrome c dramatically down-regulated NLRP3 inflammasome activation and improved the cardiomyocyte damage induced by HG, which was also detected in cells treated by Gps. Furthermore, Gps also reduced the levels of the C-reactive proteins (CRPs), IL-1β and IL-18, inhibited NLRP3 inflammasome activation and consequently improved myocardial damage in vivo. These findings provide a mechanism that ROS induced by HG activates the NLRP3 inflammasome by cytochrome c binding to NLRP3 and that Gps may be potential and effective drugs for DCM via the inhibition of ROS-mediated NLRP3 inflammasome activation.
Keywords: NLRP3 inflammasome; ROS; cytochrome c; diabetic cardiomyopathy; gypenosides; high glucose.
© 2018 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures





Similar articles
-
NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model.PLoS One. 2014 Aug 19;9(8):e104771. doi: 10.1371/journal.pone.0104771. eCollection 2014. PLoS One. 2014. PMID: 25136835 Free PMC article.
-
Activation of the TXNIP/NLRP3 inflammasome pathway contributes to inflammation in diabetic retinopathy: a novel inhibitory effect of minocycline.Inflamm Res. 2017 Feb;66(2):157-166. doi: 10.1007/s00011-016-1002-6. Epub 2016 Oct 26. Inflamm Res. 2017. PMID: 27785530
-
ALDH2 Overexpression Alleviates High Glucose-Induced Cardiotoxicity by Inhibiting NLRP3 Inflammasome Activation.J Diabetes Res. 2019 Nov 21;2019:4857921. doi: 10.1155/2019/4857921. eCollection 2019. J Diabetes Res. 2019. PMID: 31871948 Free PMC article.
-
NLRP3 Inflammasome in Diabetic Cardiomyopathy and Exercise Intervention.Int J Mol Sci. 2021 Dec 8;22(24):13228. doi: 10.3390/ijms222413228. Int J Mol Sci. 2021. PMID: 34948026 Free PMC article. Review.
-
NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases.Redox Biol. 2015;4:296-307. doi: 10.1016/j.redox.2015.01.008. Epub 2015 Jan 14. Redox Biol. 2015. PMID: 25625584 Free PMC article. Review.
Cited by
-
Association between Proinflammatory Markers, Leukocyte-Endothelium Interactions, and Carotid Intima-Media Thickness in Type 2 Diabetes: Role of Glycemic Control.J Clin Med. 2020 Aug 5;9(8):2522. doi: 10.3390/jcm9082522. J Clin Med. 2020. PMID: 32764458 Free PMC article.
-
Nutraceutical Compounds Targeting Inflammasomes in Human Diseases.Int J Mol Sci. 2020 Jul 8;21(14):4829. doi: 10.3390/ijms21144829. Int J Mol Sci. 2020. PMID: 32650482 Free PMC article. Review.
-
CircRNA DICAR as a novel endogenous regulator for diabetic cardiomyopathy and diabetic pyroptosis of cardiomyocytes.Signal Transduct Target Ther. 2023 Mar 8;8(1):99. doi: 10.1038/s41392-022-01306-2. Signal Transduct Target Ther. 2023. PMID: 36882410 Free PMC article.
-
Oxidative and endoplasmic reticulum stresses are involved in palmitic acid-induced H9c2 cell apoptosis.Biosci Rep. 2019 May 21;39(5):BSR20190225. doi: 10.1042/BSR20190225. Print 2019 May 31. Biosci Rep. 2019. PMID: 31064816 Free PMC article.
-
LanGui tea, an herbal medicine formula, protects against binge alcohol-induced acute liver injury by activating AMPK-NLRP3 signaling.Chin Med. 2024 Mar 4;19(1):41. doi: 10.1186/s13020-024-00906-0. Chin Med. 2024. PMID: 38439080 Free PMC article.
References
-
- Wei H, Qu H, Wang H, et al. 1,25‐Dihydroxyvitamin‐D3 prevents the development of diabetic cardiomyopathy in type 1 diabetic rats by enhancing autophagy via inhibiting the beta‐catenin/TCF4/GSK‐3beta/mTOR pathway. J Steroid Biochem Mol Biol. 2017;168:71‐90. - PubMed
-
- Ma ZG, Yuan YP, Xu SC, et al. CTRP3 attenuates cardiac dysfunction, inflammation, oxidative stress and cell death in diabetic cardiomyopathy in rats. Diabetologia. 2017;60:1126‐1137. - PubMed
-
- Huynh K, Bernardo BC, McMullen JR, Ritchie RH. Diabetic cardiomyopathy: mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol Ther. 2014;142:375‐415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

