Prediction of Abdominal Aortic Aneurysm Growth Using Dynamical Gaussian Process Implicit Surface
- PMID: 29993480
- PMCID: PMC6414317
- DOI: 10.1109/TBME.2018.2852306
Prediction of Abdominal Aortic Aneurysm Growth Using Dynamical Gaussian Process Implicit Surface
Abstract
Objective: We propose a novel approach to predict the Abdominal Aortic Aneurysm (AAA) growth in future time, using longitudinal computer tomography (CT) scans of AAAs that are captured at different times in a patient-specific way.
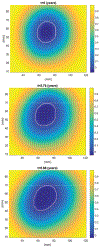
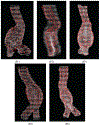
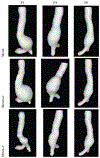
Methods: We adopt a formulation that considers a surface of the AAA as a manifold embedded in a scalar field over the three dimensional (3D) space. For this formulation, we develop our Dynamical Gaussian Process Implicit Surface (DGPIS) model based on observed surfaces of 3D AAAs as visible variables while the scalar fields are hidden. In particular, we use Gaussian process regression to construct the field as an observation model from CT training image data. We then learn a dynamic model to represent the evolution of the field. Finally, we derive the predicted AAA surface from the predicted field along with uncertainty quantified in future time.
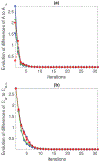
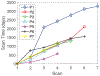
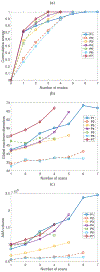
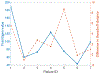
Results: A dataset of 7 subjects (4-7 scans) was collected and used to evaluate the proposed method by comparing its prediction Hausdorff distance errors against those of simple extrapolation. In addition, we evaluate the prediction results with respect to a conventional shape analysis technique such as Principal Component Analysis (PCA). All comparative results show the superior prediction performance of the proposed approach.
Conclusion: We introduce a novel approach to predict the AAA growth and its predicted uncertainty in future time, using longitudinal CT scans in a patient-specific fashion.
Significance: The capability to predict the AAA shape and its confidence region by our approach establish the potential for guiding clinicians with informed decision in conducting medical treatment and monitoring of AAAs.
Figures

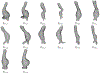



References
-
- Porth CM, Essentials of pathophysiology: Concepts of altered health states. Lippincott Williams & Wilkins, 2010.
-
- Zankl AR, Schumacher H, Krumsdorf U, Katus HA, Jahn L et al. , “Pathology, natural history and treatment of abdominal aortic aneurysms,” Clinical Research in Cardiology, vol. 96, no. 3, pp. 140–151, 2007. - PubMed
-
- Klink A, Hyafil F, Rudd J, Faries P, Fuster V, Mallat Z, Meilhac O, Mulder WJ, Michel J-B, Ramirez F et al. , “Diagnostic and thera-peutic strategies for small abdominal aortic aneurysms,” Nature Reviews Cardiology, vol. 8, no. 6, pp. 338–347, 2011. - PubMed
-
- Kniemeyer H, Kessler T, Reber PU, Ris HB, Hakki H, and Widmer MK, “Treatment of ruptured abdominal aortic aneurysm, a permanent challenge or a waste of resources? prediction of outcome using a multi-organ-dysfunction score,” European Journal of Vascular and Endovascular Surgery, pp. 190–196, 2000. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

