Hearing Changes After Intratympanic Steroids for Secondary (Salvage) Therapy of Sudden Hearing Loss: A Meta-Analysis Using Mathematical Simulations of Drug Delivery Protocols
- PMID: 29995001
- PMCID: PMC6481671
- DOI: 10.1097/MAO.0000000000001872
Hearing Changes After Intratympanic Steroids for Secondary (Salvage) Therapy of Sudden Hearing Loss: A Meta-Analysis Using Mathematical Simulations of Drug Delivery Protocols
Abstract
Objective: The use of glucocorticoids for secondary (salvage/rescue) therapy of idiopathic sudden hearing loss (ISSHL), including controlled and uncontrolled studies with intratympanic injections or continuous, catheter mediated applications, were evaluated by means of a meta-analysis in an attempt to define optimal local drug delivery protocols for ISSHL.
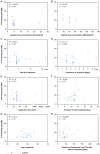
Study design: A total of 30 studies with 33 treatment groups between January 2000 and June 2014 were selected based on sufficiently detailed description of application protocols. Cochlear drug levels were calculated by a validated computer model of drug dispersion in the inner ear fluids based on the concentration and volume of glucocorticoids applied, the time drug remained in the middle ear, and on the specific timing of injections. Various factors were compared with hearing outcome, including baseline data, individual parameters of the application protocols, calculated peak concentration (Cmax), and total dose (area under the curve, AUC).
Results: There was no dependence of hearing outcome on individual parameters of the application protocol, Cmax or AUC. Hearing gain and final hearing thresholds were independent of treatment delay.
Conclusion: Based on the available data from uncontrolled and controlled randomized and non-randomized studies no clear recommendation can be made so far for a specific application protocol for either primary or secondary (salvage) intratympanic steroid treatment in patients with ISSHL. For meta-analyses, change in pure tone average (PTA) may not be an adequate outcome parameter to assess effectiveness of the intervention especially with inhomogeneity of patient populations. Final PTA might provide a better outcome parameter.
Figures





References
-
- Garavello W, Galluzzi F, Gaini RM, et al. Intratympanic steroid treatment for sudden deafness: a meta-analysis of randomized controlled trials. Otol Neurotol. 2012;33:724–9. - PubMed
-
- Hentzer E. Ultrastructure of the middle ear mucosa. Acta Otolaryngol Suppl. 1984;414:19–27. - PubMed
-
- Thompson H, Tucker AS. Dual origin of the epithelium of the mammalian middle ear. Science. 2013;339:1453–6. - PubMed
-
- Plontke SK, Mikulec AA, Salt AN. Rapid clearance of methylprednisolone after intratympanic application in humans. Comment on: Bird PA, Begg EJ, Zhang M, et al. Intratympanic versus intravenous delivery of methylprednisolone to cochlear perilymph. Otol Neurotol 2007;28:1124–30. Otol Neurotol. 2008;29:732–3. author reply 3. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

