Minimally Invasive Ponto Surgery Versus the Linear Incision Technique With Soft Tissue Preservation for Bone Conduction Hearing Implants: A Multicenter Randomized Controlled Trial
- PMID: 29995008
- PMCID: PMC6075882
- DOI: 10.1097/MAO.0000000000001852
Minimally Invasive Ponto Surgery Versus the Linear Incision Technique With Soft Tissue Preservation for Bone Conduction Hearing Implants: A Multicenter Randomized Controlled Trial
Abstract
Objective: To compare the surgical outcomes of the Minimally Invasive Ponto Surgery (MIPS) technique with those of the linear incision technique with soft-tissue preservation for bone-anchored hearing systems (BAHS).
Design: Sponsor-initiated multicenter, open, randomized, controlled clinical trial.
Setting: Maastricht University Medical Centre, Ziekenhuisgroep Twente and Medisch Centrum Leeuwarden, all situated in The Netherlands.
Participants: Sixty-four adult patients eligible for unilateral BAHS surgery.Interventions Single-stage BAHS surgery with 1:1 randomization to the linear incision technique with soft-tissue preservation (control) or the MIPS (test) group.
Primary and secondary outcome measurements: Primary objective: compare the incidence of inflammation (Holgers Index ≥ 2) during 12 weeks' follow-up after surgery. Secondary objectives: skin dehiscence, pain scores, loss of sensibility around the implant, soft-tissue overgrowth, skin sagging, implant extrusion, cosmetic results, surgical time, wound healing and Implant Stability Quotient measurements.
Results: Sixty-three subjects were analyzed in the intention-to-treat population. No significant difference was found for the incidence of inflammation between groups. Loss of skin sensibility, cosmetic outcomes, skin sagging, and surgical time were significantly better in the test group. No statistically significant differences were found for dehiscence, pain, and soft-tissue overgrowth. A nonsignificant difference in extrusion was found for the test group. The Implant Stability Quotient was statistically influenced by the surgical technique, abutment length, and time.
Conclusion: No significant differences between the MIPS and the linear incision techniques were observed regarding skin inflammation. MIPS results in a statistically significant reduction in the loss of skin sensibility, less skin sagging, improved cosmetic results, and reduced surgical time. Although nonsignificant, the implant extrusion rate warrants further research.
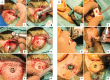
Figures



Similar articles
-
Ten years of experience with the Ponto bone-anchored hearing system-A systematic literature review.Clin Otolaryngol. 2020 Sep;45(5):667-680. doi: 10.1111/coa.13556. Epub 2020 May 25. Clin Otolaryngol. 2020. PMID: 32386454 Free PMC article.
-
Minimally Invasive Ponto Surgery compared to the linear incision technique without soft tissue reduction for bone conduction hearing implants: study protocol for a randomized controlled trial.Trials. 2016 Nov 9;17(1):540. doi: 10.1186/s13063-016-1662-0. Trials. 2016. PMID: 27829464 Free PMC article. Clinical Trial.
-
Long-Term Outcomes of the Minimally Invasive Ponto Surgery vs. Linear Incision Technique With Soft Tissue Preservation for Installation of Percutaneous Bone Conduction Devices.Front Neurol. 2021 Feb 24;12:632987. doi: 10.3389/fneur.2021.632987. eCollection 2021. Front Neurol. 2021. PMID: 33716934 Free PMC article.
-
Six-Month Clinical Outcomes for Bone-Anchored Hearing Implants: Comparison Between Minimally Invasive Ponto Surgery and the Linear Incision Technique With Tissue Preservation.Otol Neurotol. 2020 Apr;41(4):e475-e483. doi: 10.1097/MAO.0000000000002562. Otol Neurotol. 2020. PMID: 32176135
-
[Advances in minimally invasive cochlear implantation].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015 Oct;29(19):1754-8. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015. PMID: 26999856 Review. Chinese.
Cited by
-
Microbiome on the Bone-Anchored Hearing System: A Prospective Study.Front Microbiol. 2019 Apr 26;10:799. doi: 10.3389/fmicb.2019.00799. eCollection 2019. Front Microbiol. 2019. PMID: 31105654 Free PMC article.
-
Multimodal Analysis of the Tissue Response to a Bone-Anchored Hearing Implant: Presentation of a Two-Year Case Report of a Patient With Recurrent Pain, Inflammation, and Infection, Including a Systematic Literature Review.Front Cell Infect Microbiol. 2021 Mar 30;11:640899. doi: 10.3389/fcimb.2021.640899. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33859952 Free PMC article.
-
Long-Term Clinical Outcomes for Bone-Anchored Hearing Implants: 3-Year Comparison Between Minimally Invasive Ponto Surgery and the Linear Incision Technique With Tissue Preservation.Otol Neurotol. 2025 Feb 1;46(2):161-169. doi: 10.1097/MAO.0000000000004398. Otol Neurotol. 2025. PMID: 39792980 Free PMC article.
-
Ex vivo Evaluation of a New Drill System for Placement of Percutaneous Bone Conduction Devices.Front Surg. 2022 Mar 21;9:858117. doi: 10.3389/fsurg.2022.858117. eCollection 2022. Front Surg. 2022. PMID: 35388366 Free PMC article.
-
Ten years of experience with the Ponto bone-anchored hearing system-A systematic literature review.Clin Otolaryngol. 2020 Sep;45(5):667-680. doi: 10.1111/coa.13556. Epub 2020 May 25. Clin Otolaryngol. 2020. PMID: 32386454 Free PMC article.
References
-
- Crowson MG, Tucci DL. Mini review of the cost-effectiveness of unilateral osseointegrated implants in adults: Possibly cost-effective for the correct indication. Audiol Neurotol 2016; 21:69–71. - PubMed
-
- Monksfield P, Jowett S, Reid A, Proops D. Cost-effectiveness analysis of the bone-anchored hearing device. Otol Neurotol 2011; 32:1192–1197. - PubMed
-
- Stenfelt S, Goode RL. Bone-conducted sound: Physiological and clinical aspects. Otol Neurotol 2005; 26:1245–1261. - PubMed
-
- Tjellström A, Lindström J, Hallén O, Albrektsson T, Brånemark PI. Osseointegrated titanium implants in the temporal bone. A clinical study on bone-anchored hearing aids. Am J Otol 1981; 2:304–310. - PubMed
-
- Holgers KM, Tjellström A, Bjursten LM, Erlandsson BE. Soft tissue reactions around percutaneous implants: A clinical study of soft tissue conditions around skin-penetrating titanium implants for bone-anchored hearing aids. Am J Otol 1988; 9:56–59. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

