Mechanism of parkin activation by PINK1
- PMID: 29995846
- PMCID: PMC6071873
- DOI: 10.1038/s41586-018-0224-x
Mechanism of parkin activation by PINK1
Abstract
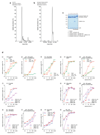
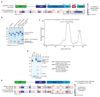
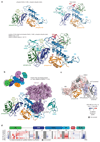
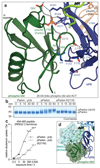
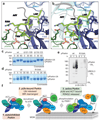
Mutations in the E3 ubiquitin ligase parkin (PARK2, also known as PRKN) and the protein kinase PINK1 (also known as PARK6) are linked to autosomal-recessive juvenile parkinsonism (AR-JP)1,2; at the cellular level, these mutations cause defects in mitophagy, the process that organizes the destruction of damaged mitochondria3,4. Parkin is autoinhibited, and requires activation by PINK1, which phosphorylates Ser65 in ubiquitin and in the parkin ubiquitin-like (Ubl) domain. Parkin binds phospho-ubiquitin, which enables efficient parkin phosphorylation; however, the enzyme remains autoinhibited with an inaccessible active site5,6. It is unclear how phosphorylation of parkin activates the molecule. Here we follow the activation of full-length human parkin by hydrogen-deuterium exchange mass spectrometry, and reveal large-scale domain rearrangement in the activation process, during which the phospho-Ubl rebinds to the parkin core and releases the catalytic RING2 domain. A 1.8 Å crystal structure of phosphorylated human parkin reveals the binding site of the phospho-Ubl on the unique parkin domain (UPD), involving a phosphate-binding pocket lined by AR-JP mutations. Notably, a conserved linker region between Ubl and the UPD acts as an activating element (ACT) that contributes to RING2 release by mimicking RING2 interactions on the UPD, explaining further AR-JP mutations. Our data show how autoinhibition in parkin is resolved, and suggest a mechanism for how parkin ubiquitinates its substrates via an untethered RING2 domain. These findings open new avenues for the design of parkin activators for clinical use.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

