Evolution of a central neural circuit underlies Drosophila mate preferences
- PMID: 29995860
- PMCID: PMC6276375
- DOI: 10.1038/s41586-018-0322-9
Evolution of a central neural circuit underlies Drosophila mate preferences
Abstract
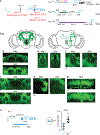
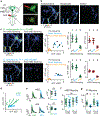
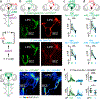
Courtship rituals serve to reinforce reproductive barriers between closely related species. Drosophila melanogaster and Drosophila simulans exhibit reproductive isolation, owing in part to the fact that D. melanogaster females produce 7,11-heptacosadiene, a pheromone that promotes courtship in D. melanogaster males but suppresses courtship in D. simulans males. Here we compare pheromone-processing pathways in D. melanogaster and D. simulans males to define how these sister species endow 7,11-heptacosadiene with the opposite behavioural valence to underlie species discrimination. We show that males of both species detect 7,11-heptacosadiene using homologous peripheral sensory neurons, but this signal is differentially propagated to P1 neurons, which control courtship behaviour. A change in the balance of excitation and inhibition onto courtship-promoting neurons transforms an excitatory pheromonal cue in D. melanogaster into an inhibitory cue in D. simulans. Our results reveal how species-specific pheromone responses can emerge from conservation of peripheral detection mechanisms and diversification of central circuitry, and demonstrate how flexible nodes in neural circuits can contribute to behavioural evolution.
Figures




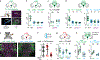

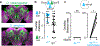
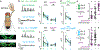

Comment in
-
Neural circuit evolved to process pheromone differently in two species of fruit fly.Nature. 2018 Jul;559(7715):485-487. doi: 10.1038/d41586-018-05595-y. Nature. 2018. PMID: 30030513 No abstract available.
References
-
- Lim MM et al. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 429, 754–757 (2004). - PubMed
-
- Weber JN, Peterson BK & Hoekstra HE Discrete genetic modules are responsible for complex burrow evolution in Peromyscus mice. Nature 493, 402–405 (2013). - PubMed
-
- Ding Y, Berrocal A, Morita T, Longden KD & Stern DL Natural courtship song variation caused by an intronic retroelement in an ion channel gene. Nature 536, 329–332 (2016). - PubMed
-
- Hey J & Kilman RM Population genetics and phylogenetics of DNA sequence variation at multiple loci within the Drosophila melanogaster species complex. Mol. Biol. Evol. 10, 804–822 (1993). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

