Clobazam monotherapy for focal or generalized seizures
- PMID: 29995989
- PMCID: PMC6513499
- DOI: 10.1002/14651858.CD009258.pub3
Clobazam monotherapy for focal or generalized seizures
Abstract
Background: This is an updated version of the original Cochrane Review published in Issue 10, 2014. There is a need to expand monotherapy options available to a clinician for the treatment of new focal or generalized seizures. A Cochrane systematic review for clobazam monotherapy is expected to define its place in the treatment of new-onset or untreated seizures and highlight gaps in evidence.
Objectives: To evaluate the efficacy, effectiveness, tolerability and safety of clobazam as monotherapy in people with new-onset focal or generalized seizures.
Search methods: For the latest update we searched the following databases on 19 March 2018: the Cochrane Register of Studies (CRS Web), which includes the Cochrane Epilepsy Group Specialized Register and the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (Ovid, 1946- ), BIOSIS Previews (1969- ), ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform (ICTRP). There were no language restrictions.
Selection criteria: Randomized or quasi-randomized controlled trials comparing clobazam monotherapy versus placebo or other anti-seizure medication in people with two or more unprovoked seizures or single acute symptomatic seizure requiring short-term continuous anti-seizure medication, were eligible for inclusion.
Data collection and analysis: Primary outcome measure was time on allocated treatment (retention time), reflecting both efficacy and tolerability. Secondary outcomes included short- and long-term effectiveness measures, tolerability, quality of life, and tolerance measures. Two authors independently extracted the data.
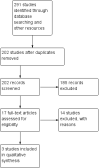
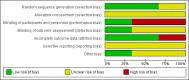
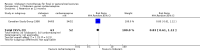
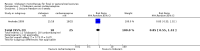
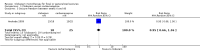
Main results: We identified three trials fulfilling the review criteria, which included 206 participants. None of the identified studies reported the preselected primary outcome measure. A meta-analysis was not possible. Lack of detail regarding allocation concealment and a high risk of performance and detection bias in two studies prompted us to downgrade the quality of evidence (by using the GRADE approach) for some of our results due to risk of bias.Regarding retention at 12 months, we detected no evidence of a statistically significant difference between clobazam and carbamazepine (risk ratio (RR) 0.83, 95% confidence interval (CI) 0.61 to 1.12; low-quality evidence). There was low-quality evidence that clobazam led to better retention compared with phenytoin (RR 1.43, 95% CI 1.08 to 1.90). We could not determine whether participants receiving clobazam were found to be less likely to discontinue it due to adverse effects as compared to phenytoin (RR 0.10, 95% CI 0.01 to 1.65, low-quality evidence).
Authors' conclusions: We found no advantage for clobazam over carbamazepine for retention at 12 months in drug-naive children and a slight advantage of clobazam over phenytoin for retention at six months in adolescents and adults with neurocysticercosis in a single clinical trial each. At present, the available evidence is insufficient to inform clinical practice.
Conflict of interest statement
Ravindra Arya receives research support (total 5% effort) from Pediatric Epilepsy Research Foundation, which is unrelated to the present work Vidhu Anand: none known Nisha Giridharan: none known Sushil K Garg: none known
Figures
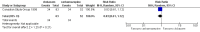
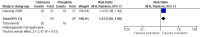









Update of
-
Clobazam monotherapy for partial-onset or generalized-onset seizures.Cochrane Database Syst Rev. 2014 Oct 4;(10):CD009258. doi: 10.1002/14651858.CD009258.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2018 Jul 11;7:CD009258. doi: 10.1002/14651858.CD009258.pub3. PMID: 25280512 Updated.
References
References to studies included in this review
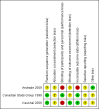
Andrade 2009 {published data only}
-
- Andrade R, García‐Espinosa A, Machado‐Rojas A, García‐González ME, Trápaga‐Quincoses O, Morales‐Chacón LM. A prospective, open, controlled and randomised study of clobazam versus carbamazepine in patients with frequent episodes of Rolandic epilepsy [Estudio prospectivo, abierto, controlado y aleatorizado de clobazam frente a carbamacepina en pacientes con crisis frecuentes de epilepsia Rolándica]. Revista de Neurologia 2009;49(11):581‐6. - PubMed
Canadian Study Group 1998 {published data only}
-
- Canadian Study Group for Childhood Epilepsy. Clobazam has equivalent efficacy to carbamazepine and phenytoin as monotherapy for childhood epilepsy. Epilepsia 1998;39(9):952‐9. [PUBMED: 9738674] - PubMed
Kaushal 2006 {published data only}
-
- Kaushal S, Rani A, Chopra SC, Singh G. Safety and efficacy of clobazam versus phenytoin‐sodium in the antiepileptic drug treatment of solitary cysticercus granulomas. Neurology India 2006;54(2):157‐60. [PUBMED: 16804259] - PubMed
References to studies excluded from this review
Bajaj 2005 {published data only}
-
- Bajaj AS, Bajaj BK, Puri V, Tayal G. Intermittent clobazam in febrile seizures: an Indian experience. Journal of Pediatric Neurology 2005;3(1):19‐23.
Canadian Study Group 1999 {published data only}
-
- Bawden HN, Camfield CS, Camfield PR, Cunningham C, Darwish H, Dooley JM, et al. The cognitive and behavioural effects of clobazam and standard monotherapy are comparable; Canadian Study Group for Childhood Epilepsy. Epilepsy Research 1999;33(2‐3):133‐43. [PUBMED: 10094425] - PubMed
Feely 1982a {published data only}
-
- Feely M, Calvert R, Gibson J. Clobazam in catamenial epilepsy. A model for evaluating anticonvulsants. Lancet 1982;2(8289):71‐3. [PUBMED: 6123810] - PubMed
Feely 1982b {published data only}
-
- Feely M, Calvert R, Gibson J. Catamenial epilepsy as a model for testing a new anticonvulsant (clobazam). Irish Journal of Medical Science 1982;151(11):358‐9.
Feely 1982c {published data only}
-
- Feely M, Calvert R, Gibson J. Clobazam in the treatment of catamenial epilepsy. British Journal of Clinical Pharmacology 1982;13(2):273P‐4P. [Abstract: Proceedings of the British Pharmacological Society; 1981 Sept 16‐18; University of Oxford, Oxford, UK]
Feely 1984 {published data only}
Figueroa 1984 {published data only}
-
- Figueroa D, Adlerstein L, Manterola A. Clobazam in refractory epilepsies of children [Clobazam en epilepsias refractarias del nino]. Revista Chilena de Pediatria 1984;55(6):401‐5. [PUBMED: 6399391] - PubMed
Khosroshahi 2011 {published data only}
-
- Khosroshahi N, Faramarzi F, Salamati P, Haghighi SM, Kamrani K. Diazepam versus clobazam for intermittent prophylaxis of febrile seizures. Indian Journal of Pediatrics 2011;78(1):38‐40. [PUBMED: 20890683] - PubMed
Koeppen 1987 {published data only}
-
- Koeppen D, Baruzzi A, Capozza M, Chauvel P, Courjon J, Favel P, et al. Clobazam in therapy‐resistant patients with partial epilepsy: a double‐blind placebo‐controlled crossover study. Epilepsia 1987;28(5):495‐506. [PUBMED: 3115770] - PubMed
RamachandranNair 2005 {published data only}
-
- Ramachandran Nair R, Parameswaran M, Girija AS. Intermittent oral prophylaxis in preventing recurrent febrile seizures, diazepam versus clobazam: a randomised study in south Indian rural children. Epilepsia 2005;46(Suppl 6):240.
Rose 2005 {published data only}
-
- Rose W, Kirubakaran C, Scott JX. Intermittent clobazam therapy in febrile seizures. Indian Journal of Pediatrics 2005;72(1):31‐3. [PUBMED: 15684445] - PubMed
Vajda 1985 {published data only}
-
- Vajda FJ, Bladin PF, Parsons BJ. Clinical experience with clobazam: a new 1,5 benzodiazepine in the treatment of refractory epilepsy. Clinical and Experimental Neurology 1985;21:177‐82. [PUBMED: 3843217] - PubMed
Yagi 1995 {published data only}
-
- Yagi K, Takeda A, Kawai I. The clinical efficacy of nh‐15 (clobazam) for the treatment refractory epilepsy by the double‐blind comparative study with inactive placebo. Igakunoayumi 1995;174(3):229‐41.
Yamatogi 1997 {published data only}
-
- Yamatogi Y, Ohtahara S, Shigematsu H, Oguni H, Nakashima M. Single blind comparative study of clobazam (NH‐15) with clonazepam in intractable childhood epilepsies. Journal of the Japan Epilepsy Society 1997;15(2):110‐21.
Additional references
Banerjee 2008
-
- Banerjee PN, Hauser WA. Incidence and Prevalence. In: Engel Jr J, Pedley TA editor(s). Epilepsy. A Comprehensive Textbook. 2nd Edition. Vol. 1, Philadelphia: Lippincott Williams & Wilkins, 2008:45‐56.
Bucher 1997
-
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐ analysis of randomised controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. - PubMed
Canadian Clobazam Cooperative Group 1991
-
- Canadian Clobazam Cooperative Group. Clobazam in treatment of refractory epilepsy: the Canadian experience. A retrospective study. Epilepsia 1991;32(3):407‐16. - PubMed
Commission 1998
-
- The ILAE Commission on Antiepileptic Drugs. Considerations on designing clinical trials to evaluate the place of new antiepileptic drugs in the treatment of newly diagnosed and chronic patients with epilepsy. Epilepsia 1998;39(7):799‐803. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Engel 2008
-
- Engel J Jr, Pedley TA. Introduction: What is Epilepsy?. In: Engel J Jr, Pedley TA editor(s). Epilepsy. A Comprehensive Textbook. 2nd Edition. Vol. 1, Philadelphia: Lippincott Williams & Wilkins, 2008:1‐7.
Fisher 2005
-
- Fisher RS, Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy. Definitions proposed by the International League against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005;46(4):470‐2. - PubMed
Glauser 2006
-
- Glauser T, Ben‐Menachem E, Bourgeois B, Cnaan A, Chadwick D, Guerreiro C, et al. ILAE Treatment Guidelines: Evidence‐based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia 2006;47(7):1094‐120. - PubMed
Glauser 2013
-
- Glauser T, Ben‐Menachem E, Bourgeois B, Cnaan A, Guerreiro C, Kalviainen R, et al. for the ILAE subcommission of AED Guidelines. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia 2013;54(3):551‐63. [PUBMED: 23350722] - PubMed
Higgins 2003
Higgins 2011a
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. Cochrane Collaboration.
Higgins 2011b
-
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Löscher 1987
-
- Löscher W, Schmidt D. Diazepam increases aminobutyric acid in human cerebrospinal fluid. Journal of Neurochemistry 1987;49:152‐7. - PubMed
Macdonald 2002
-
- Macdonald R. Benzodiazepines. Mechanism of Action. In: Levy R, Mattson R, Meldrum B, et al. editor(s). Antiepileptic Drugs. 5th Edition. Philadelphia: Lippincott, Williams & Wilkins, 2002:179‐86.
Murray 1994
-
- Murray CGL, Lopez AD. Global comparative assessment in the Health Sector; Disease Burden, Expenditures, and Intervention Packages. Geneva: WHO, 1994.
Porter 2007
-
- Porter RJ, Meldrum BS. Antiseizure Drugs. In: Katzung BG editor(s). Basic and Clinical Pharmacology. 10th Edition. Mc Graw Hill, 2007:374‐94.
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rogawski 2004
-
- Rogawski MA, Löscher W. The neurobiology of antiepileptic drugs. Nature Reviews Neuroscience 2004;5(1):1‐12. - PubMed
Schmidt 1986
-
- Schmidt D, Rohde M, Wolf P, Roeder‐Wanner U. Clobazam for refractory focal epilepsy. A controlled trial. Archives of Neurology 1986;43(8):824‐6. - PubMed
Schmidt 2008
-
- Schmidt D, Wilensky AJ. Benzodiazepines. In: Engel J Jr, Pedley TA editor(s). Epilepsy. A Comprehensive Textbook. 2nd Edition. Vol. 2, Philadelphia: Lippincott Williams & Wilkins, 2008:1531‐41.
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chaper 12: Interpreting results and drawing conclusions. In Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated September 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
References to other published versions of this review
Arya 2011
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

