A Neural Circuit Underlying the Generation of Hot Flushes
- PMID: 29996088
- PMCID: PMC6094949
- DOI: 10.1016/j.celrep.2018.06.037
A Neural Circuit Underlying the Generation of Hot Flushes
Abstract
Hot flushes are a sudden feeling of warmth commonly associated with the decline of gonadal hormones at menopause. Neurons in the arcuate nucleus of the hypothalamus that express kisspeptin and neurokinin B (Kiss1ARH neurons) are candidates for mediating hot flushes because they are negatively regulated by sex hormones. We used a combination of genetic and viral technologies in mice to demonstrate that artificial activation of Kiss1ARH neurons evokes a heat-dissipation response resulting in vasodilation (flushing) and a corresponding reduction of core-body temperature in both females and males. This response is sensitized by ovariectomy. Brief activation of Kiss1ARH axon terminals in the preoptic area of the hypothalamus recapitulates this response, while pharmacological blockade of neurokinin B (NkB) receptors in the same brain region abolishes it. We conclude that transient activation of Kiss1ARH neurons following sex-hormone withdrawal contributes to the occurrence of hot flushes via NkB release in the rostral preoptic area.
Keywords: chemogenetics; estrogen; hot flashes; kisspeptin; menopause; neurokinin B; optogenetics; temperature regulation.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures

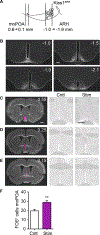

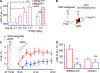
References
-
- Casper RF, Yen SS, and Wilkes MM (1979). Menopausal flushes: a neuroendocrine link with pulsatile luteninizing hormone secreation. Science 205, 823–825. - PubMed
-
- de Croft S, Boehm U, and Herbison AE (2013). Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology 154, 2750–2760. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

