Complexation of the Mycotoxin Cyclopiazonic Acid with Lanthanides Yields Luminescent Products
- PMID: 29996475
- PMCID: PMC6071049
- DOI: 10.3390/toxins10070285
Complexation of the Mycotoxin Cyclopiazonic Acid with Lanthanides Yields Luminescent Products
Abstract
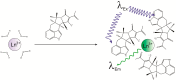
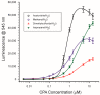
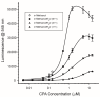
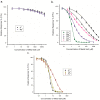
Cycopiazonic acid (CPA) is a neurotoxin that acts through inhibition of the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA). CPA blocks the calcium access channel of the enzyme. The inhibition may involve the binding of CPA with a divalent cation such as Mg2+. The potential for CPA to act as a chelator also has implications for methods to detect this toxin. Certain of the lanthanide metals undergo a dramatic increase in luminescence upon coordination with small molecules that can transfer excitation energy to the metal. This report is the first to describe the coordination of CPA with lanthanide metals, resulting in a substantial enhancement of their luminescence. The luminescence expressed was dependent upon the type of lanthanide, its concentration, and the environment (solvent, water content, pH). Based upon the phenomenon, a competitive assay was also developed wherein terbium (Tb3+) and a series of metal cations competed for binding with CPA. With increasing cation concentration, the luminescence of the CPA/Tb3+ complex was inhibited. The chlorides of ten metals were tested. Inhibition was best with Cu2+, followed by Co2+, Al3+, Fe3+, Mn2+, Au3+, Mg2+, and Ca2+. Two cations in oxidation state one (Na⁺, K⁺) did not inhibit the interaction significantly. The interaction of CPA with lanthanides provides a novel recognition assay for this toxin. It also provides a novel way to probe the binding of CPA to metals, giving insights into CPA’s mechanism of action.
Keywords: calcium-ATPase; cyclopiazonic acid; lanthanides; luminescence; mechanism of action; mycotoxin.
Conflict of interest statement
The author declares no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures





References
-
- Ostry V., Toman J., Grosse Y., Malir F. Cyclopiazonic acid: 50th anniversary of its discovery. World Mycotoxin J. 2018;11:135–148. doi: 10.3920/WMJ2017.2243. - DOI
-
- Dorner J.W. Recent advances in analytical methodology for cyclopiazonic acid. In: DeVries J., Trucksess M.W., Jackson L.S., editors. Mycotoxins in Food Safety. Kluwer Academic/Plenum Publishers; New York, NY, USA: 2002. pp. 107–116. - PubMed
-
- Trucksess M.W., Mislivec P.P., Young K., Bruce V.R., Page S.W. Cyclopiazonic acid production by cultures of Aspergillus and Penicillium species isolated from dried beans, corn meal, macaroni, and pecans. J. AOAC Int. 1987;70:123–126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

