Highly Sensitive Acetone Gas Sensor Based on g-C₃N₄ Decorated MgFe₂O₄ Porous Microspheres Composites
- PMID: 29996480
- PMCID: PMC6068867
- DOI: 10.3390/s18072211
Highly Sensitive Acetone Gas Sensor Based on g-C₃N₄ Decorated MgFe₂O₄ Porous Microspheres Composites
Abstract
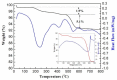
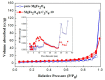
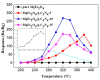
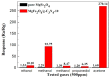
The g-C₃N₄ decorated magnesium ferrite (MgFe₂O₄) porous microspheres composites were successfully obtained via a one-step solvothermal method. The structure and morphology of the as-prepared MgFe₂O₄/g-C₃N₄ composites were characterized by the techniques of X-ray diffraction (XRD), field-emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), thermal gravity and differential scanning calorimeter (TG⁻DSC) and N₂-sorption. The gas sensing properties of the samples were measured and compared with a pure MgFe₂O₄-based sensor. The maximum response of the sensor based on MgFe₂O₄/g-C₃N₄ composites with 10 wt % g-C₃N₄ content to acetone is improved by about 145 times, while the optimum temperature was lowered by 60 °C. Moreover, the sensing mechanism and the reason for improving gas sensing performance were also discussed.
Keywords: MgFe2O4 porous microspheres; acetone; composites; g-C3N4 nanosheet; gas sensing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Wang X.-F., Ma W., Jiang F., Cao E.-S., Sun K.-M., Cheng L., Song X.-Z. Prussian blue analogue derived porous NiFe2O4 nanotubes for low-concentration acetone sensing at low working temperature. Chem. Eng. J. 2018;338:504–512. doi: 10.1016/j.cej.2018.01.072. - DOI
-
- Ueta I., Saito Y., Hosoe M., Okamoto M., Ohkita H., Shirai S., Tamura H., Jinno K. Breath acetone analysis with miniaturized sample preparation device: In-needle preconcentration and subsequent determination by gas chromatography–mass spectroscopy. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009;877:2551–2556. doi: 10.1016/j.jchromb.2009.06.039. - DOI - PubMed
-
- Li Y., Luo N., Sun G., Zhang B., Ma G., Jin H., Wang Y., Cao J., Zhang Z. Facile synthesis of ZnFe2O4/α-Fe2O3 porous microrods with enhanced tea-sensing performance. J. Alloys Compd. 2018;737:255–262. doi: 10.1016/j.jallcom.2017.12.068. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

