Respiratory Tract Deposition and Distribution Pattern of Microparticles in Mice Using Different Pulmonary Delivery Techniques
- PMID: 29996506
- PMCID: PMC6161314
- DOI: 10.3390/vaccines6030041
Respiratory Tract Deposition and Distribution Pattern of Microparticles in Mice Using Different Pulmonary Delivery Techniques
Abstract
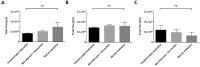
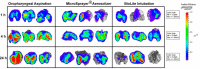
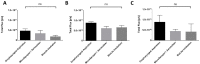
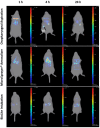
Pulmonary delivery of drugs and vaccines is an established route of administration, with particulate-based carriers becoming an attractive strategy to enhance the benefits of pulmonary therapeutic delivery. Despite the increasing number of publications using the pulmonary route of delivery, the lack of effective and uniform administration techniques in preclinical models generally results in poor translational success. In this study, we used the IVIS Spectrum small-animal in vivo imaging system to compare the respiratory tract deposition and distribution pattern of a microsphere suspension (5 µm) in mice after 1, 4, and 24 h when delivered by oropharyngeal aspiration, the Microsprayer® Aerosolizer, and the BioLite Intubation System, three-widely reported preclinical inhalation techniques. We saw no significant differences in microsphere deposition in whole body images and excised lungs (at 1, 4, and 24 h); however, the three-dimensional (3D) images showed more localized deposition in the lungs with the MicroSprayer® and BioLite delivery techniques. Further, oropharyngeal aspiration (at 1 h) showed microsphere deposition in the oral cavity, in contrast to the MicroSprayer® and BioLite systems. The studies shown here will allow researchers to choose the appropriate pulmonary delivery method in animal models based on their study requirements.
Keywords: BioLite intubation; IVIS; MicroSprayer® Aerosolizer; microparticles; oropharyngeal aspiration; pulmonary delivery; respiratory tract deposition; vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources

