Low dose mycophenolate mofetil versus cyclophosphamide in the induction therapy of lupus nephritis in Nepalese population: a randomized control trial
- PMID: 29996800
- PMCID: PMC6042432
- DOI: 10.1186/s12882-018-0973-7
Low dose mycophenolate mofetil versus cyclophosphamide in the induction therapy of lupus nephritis in Nepalese population: a randomized control trial
Abstract
Background: The management of proliferative lupus nephritis (LN) comprises timely and coordinated immunosuppressive therapy. This study aimed to evaluate and compare the effectiveness and safety profile of low dose mycophenolate mofetil (MMF) and cyclophosphamide (CYC) in induction therapy of LN in Nepalese population.
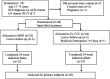
Methods: We conducted a prospective, open-label, randomized trial over a period of one and half years. Forty-nine patients with class III to V lupus nephritis were enrolled, out of which 42 patients (21 in each group) could complete the study. CYC was given intravenously as a monthly pulse and MMF was administered orally in the tablet form in the maximum daily dose of 1.5 g in two divided doses.
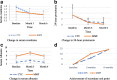
Results: The mean age of the patients was 25.43 ± 10.17 years with female to male ratio of 7.3:1. Mean baseline serum creatinine was 1.58 ± 1.38 mg/dL and eGFR was 62.38 ± 26.76 ml/min/1.73m2. Mean 24-h urinary protein was 4.35 ± 3.71 g per 1.73 m2 body surface area. At 6 months, serum creatinine (mg/dL) decreased from 1.73 to 0.96 in CYC and from 1.24 to 0.91 in the MMF group with improvement in eGFR (ml/min/1.73 m2) from 60.33 to 88.52 in CYC and from 64.42 to 89.09 in MMF group. Twenty-four-hour urinary protein (gm/1.73m2) reduced from 4.47 to 0.94 in CYC and from 4.5 to 0.62 in the MMF group. Primary end point was achieved in higher percentage of patients with MMF than CYC (28.6% vs. 19%) while equal proportion of patients (67% in each group) achieved secondary end point in both groups. Number of non-responders was higher in CYC group than in the MMF group (14.3% vs. 4.8%). There was no difference in the rate of achievement of secondary end point in both CYC and MMF groups (3.16 vs. 3.05 months). The occurrence of adverse events was higher in the CYC than in MMF group (56 vs. 15 events).
Conclusion: Present study has concluded that MMF, used in relatively lower dose, is equally effective in inducing remission with reduction of proteinuria and improvement of kidney function with lesser adverse events than CYC in the induction therapy of proliferative lupus nephritis.
Trial registration: Retrospectively registered to ClinicalTrials.gov PRS. NCT03200002 (Registered date: June 28, 2017).
Keywords: Cyclophosphamide; Induction therapy; Lupus nephritis; Mycophenolate mofetil.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval was obtained from the Institutional Review Board of National Academy of Medical Sciences (NAMS), Kathmandu, Nepal. Patients gave informed written consent to participate in the study. Written informed consent was also obtained from the parents of the participants below 18 years of age.
Consent for publication
Not applicable.
Competing interests
AS is employed by Chitwan Medical College and does not have financial benefits in participating in the study.
RH, RKA and AB are employed by the Government of Nepal and currently posted at National Academy of Medical Sciences and have not received any financial benefits for getting involved in the study.
GRB is employed by UnitedHealth Group and also owns company stocks. No financial benefits are received in participating in this study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Comparative efficacy and safety of mycophenolate mofetil and cyclophosphamide in the induction treatment of lupus nephritis: A systematic review and meta-analysis.Medicine (Baltimore). 2020 Sep 18;99(38):e22328. doi: 10.1097/MD.0000000000022328. Medicine (Baltimore). 2020. PMID: 32957400 Free PMC article.
-
Comparison of low-dose intravenous cyclophosphamide with oral mycophenolate mofetil in the treatment of lupus nephritis.Kidney Int. 2016 Jan;89(1):235-42. doi: 10.1038/ki.2015.318. Epub 2016 Jan 4. Kidney Int. 2016. PMID: 26489028 Clinical Trial.
-
Mizoribine versus mycophenolate mofetil or intravenous cyclophosphamide for induction treatment of active lupus nephritis.Chin Med J (Engl). 2014;127(21):3718-23. Chin Med J (Engl). 2014. PMID: 25382325 Clinical Trial.
-
Mycophenolate mofetil or cyclophosphamide in indian patients with lupus nephritis: Which is better? A single-center experience.Saudi J Kidney Dis Transpl. 2017 Sep-Oct;28(5):1069-1077. doi: 10.4103/1319-2442.215147. Saudi J Kidney Dis Transpl. 2017. PMID: 28937065 Clinical Trial.
-
Eurolupus cyclophosphamide plus repeated pulses of methyl-prednisolone for the induction therapy of class III, IV and V lupus nephritis.Autoimmun Rev. 2021 Oct;20(10):102898. doi: 10.1016/j.autrev.2021.102898. Epub 2021 Jul 15. Autoimmun Rev. 2021. PMID: 34274543 Review.
Cited by
-
What Have We Learnt About the Treatment of Juvenile-Onset Systemic Lupus Erythematous Since Development of the SHARE Recommendations 2012?Front Pediatr. 2022 Apr 14;10:884634. doi: 10.3389/fped.2022.884634. eCollection 2022. Front Pediatr. 2022. PMID: 35498799 Free PMC article. Review.
-
Management of lupus nephritis: a systematic literature review informing the 2019 update of the joint EULAR and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations.RMD Open. 2020 Jul;6(2):e001263. doi: 10.1136/rmdopen-2020-001263. RMD Open. 2020. PMID: 32699043 Free PMC article.
-
Comparative efficacy and safety of mycophenolate mofetil and cyclophosphamide in the induction treatment of lupus nephritis: A systematic review and meta-analysis.Medicine (Baltimore). 2020 Sep 18;99(38):e22328. doi: 10.1097/MD.0000000000022328. Medicine (Baltimore). 2020. PMID: 32957400 Free PMC article.
-
Clinical efficacy and safety of rituximab in lupus nephritis.Drug Des Devel Ther. 2019 Mar 11;13:845-856. doi: 10.2147/DDDT.S195113. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 30880917 Free PMC article.
-
The efficacy of immunosuppressive drugs induction therapy for lupus nephritis: a systematic review and network meta-analysis.Ren Fail. 2023;45(2):2290365. doi: 10.1080/0886022X.2023.2290365. Epub 2023 Dec 12. Ren Fail. 2023. PMID: 38087473 Free PMC article.
References
-
- Gourley MF, Austin HA, 3rd, Scott D, Yarboro CH, Vaughan EM, Muir J, et al. Methylprednisolone and cyclophosphamide, alone or in combination, in patients with lupus nephritis. A randomized, controlled trial. Ann Intern Med. 1996;125:549–557. doi: 10.7326/0003-4819-125-7-199610010-00003. - DOI - PubMed
-
- Houssiau FA, Vasconcelos C, D’Cruz D, Sebastiani GD, Garrido Ed Ede R, Danieli MG, et al. Immunosuppressive therapy in lupus nephritis: the euro-lupus nephritis trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002;46:2121–2131. doi: 10.1002/art.10461. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

